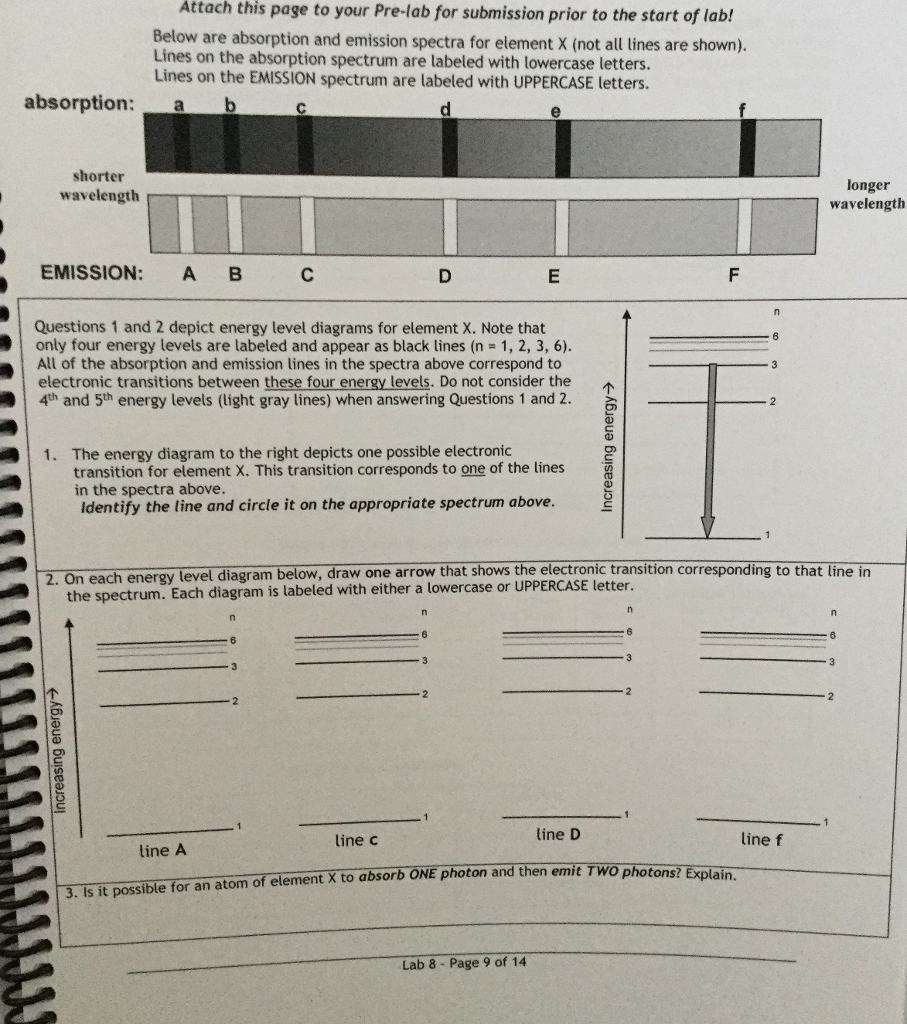
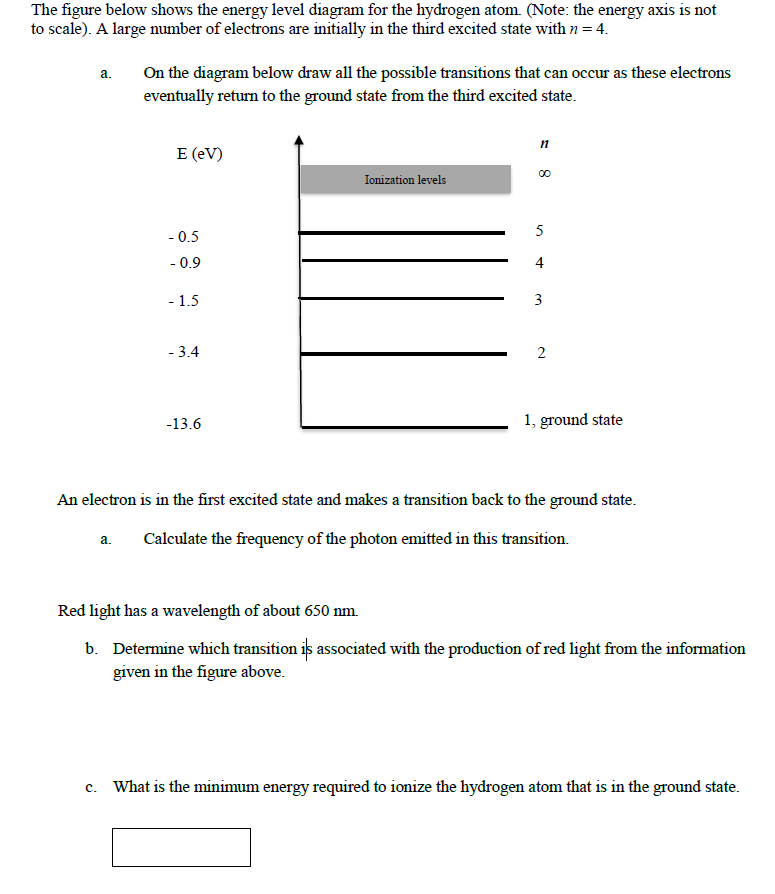
35 the figure is an energy-level diagram for a simple atom. (figure 1)
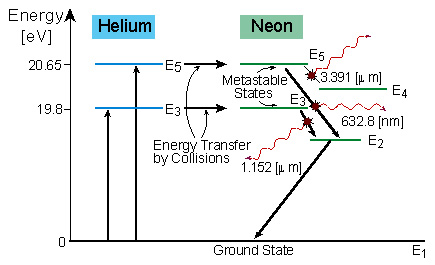
Vibrational levels v Rotational levels J United atom approximation n=1 Figure 3. Example of an energy level diagram of a diatomic molecule. transitions with upper quantum number n 3 are indicated by an arrow and labelled with the corresponding wavelength. Radiation in the visible spectral range mostly originates from transitions between excited ... The figure shows energy level diagram of hydrogen atom. (i) Find out the transition which results in the emission of a photon of wavelength 496 nm. (ii) Which transition corresponds to the emission of radiation of maximum wavelength ? Justify your answer.
PhysicsLAB: Energy-Level Diagrams. Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another. These energies differences correspond to the wavelengths of light in the discreet spectral lines emitted by an atom as it goes through de-excitation or by the ...

The figure is an energy-level diagram for a simple atom. (figure 1)
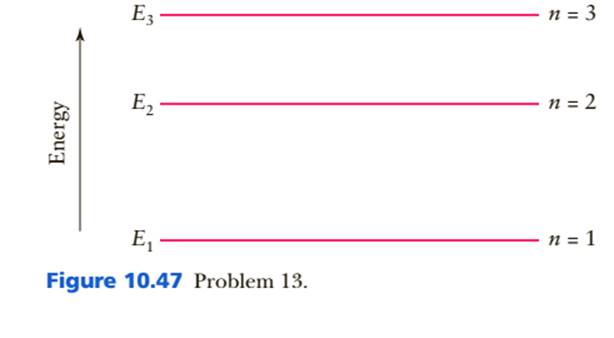
4) The figure shows part of the energy level diagram of a certain atom. The energy spacing between levels 1 and 2 is twice that between 2 and 3. If an electron makes a transition from level 3 to level 2, the radiation of wavelength λ is emitted. Visualising energy levels . In an atom, electrons around a central nucleus can only have particular energy values. These discrete values are termed 'energy levels'. In a diagram they are represented by horizontal lines, with the lowest level (the ground state) at the bottom and the highest level (ionisation) at the top. The levels are a consequence of the wave nature of electrons, as described ... Calculate the wavelength associated with the following energy transitions: (d) n = 3 to n = 2 (e) n = 2 to n = 1 (f) n = 3 to n = 1 _____ 2) The figure above shows the energy-level diagram for a hypothetical simple atom. The wavelength of the radiation emitted when an electron undergoes transition B is 400 nm (or 400 x 10-9 m), and for ...
The figure is an energy-level diagram for a simple atom. (figure 1). For a neutral atom, H= XZ j=1 ~2 2m r2 j 1 4ˇ 0 Ze2 rj! + 1 2 1 4ˇ 0! Z X j6=k e2 jrj rkj: ... Figure 5.2 - Energy level diagram for He (relative to He+, -54.4 eV). Note that parahelium (antisymmetric ... Let us consider two simple models for the conduction electron e.s. Sommerfeld's free electron gas Click here👆to get an answer to your question ️ The energy level diagram for an hydrogen like atom is shown in the figure.The radius of its first Bohr orbit is 0 eV n = ∞ - 6.04 eV n = 3 - 13.6 eV n = 2 - 54.4 eV n = 1 The diagram above shows part of an energy-level diagram for a certain atom. The wavelength of the radiation associated with transition A is 600 nm (1 nm = 1 x 10 -9 m) and that associated with transition B is 300 nm. a. Determine the energy of a photon associated with transition A. b. Determine the λ of the radiation associated with transition C Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states.
Figure Ex38.22 is an energy-level diagram for a simple atom, inwhich E1 = 0.0 eV, E2 =1.2 eV, and E3 =3.3eV. Calculate the wavelength(s)which appear(s) in the following two cases, in each of which youshould submit all of the wavelength(s) in the singleanswer box,separated by spaces.Figure Ex38.22(a) the atom's emission spectrum nm(b) the atom's ... An energy-level diagram for a hypothetical atom is shown above. a. Determine the frequency of the lowest energy photon that could ionize the atom, initially in its ground state. b. Assume the atom has been excited to the state at -1.0 electron volt. i. Determine the wavelength of the photon for each possible spontaneous transition. ii. item select "Eigenvalue" Æ "Level Diagram". You should see an energy level diagram with two energy levels above and below a dotted line (similar to the one in Figure 5). The dotted line corresponds to α, the energy of the 2py atomic orbitals. The lower level is labeled with an energy of 1.00 and this corresponds to α + 1.00β. The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV. When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon.
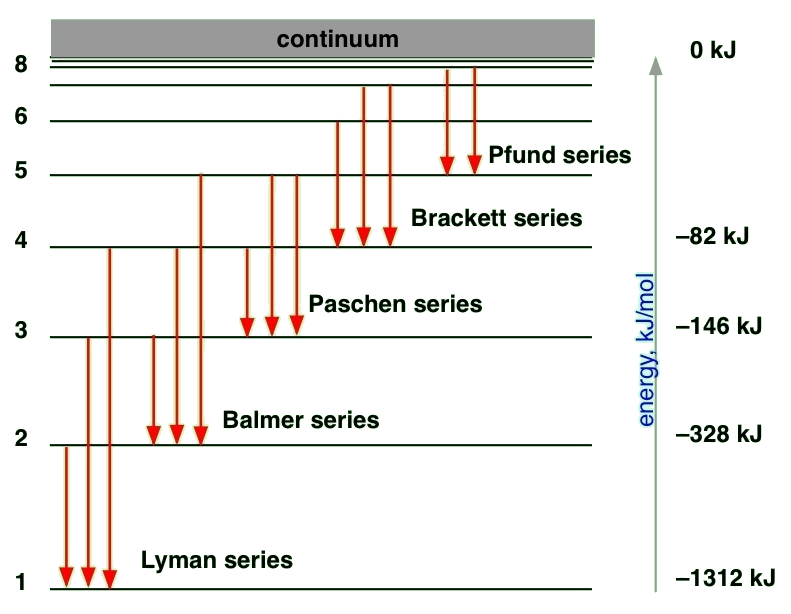
Refer to Figure 1 when answering the first 7 questions of this exam. 1. Which series of electron transitions in the energy-level diagram for Hydrogen produce the lines shown in the absorption-line spectrum of Hydrogen? ANSWER. Series #2 2. Which series of electron transitions in the energy-level diagram produce the "Balmer" 2- 1 149 (very long) 0 O2 2 120.7 2 The bond distance in N2 2- is very close to the expected bond distance for a diatomic with 12 valence electrons, as shown in Figure 5.8. 5.6 The energy level pattern would be similar to the one shown in Figure 5.5, with the interacting orbitals the 3s and 3p rather than 2s and 2p. Imgur. The energy of the electron of a monoelectronic atom depends only on which shell the electron orbits in. The energy level of the electron of a hydrogen atom is given by the following formula, where. n. n n denotes the principal quantum number: E n = − 1312 n 2 kJ/mol. E_n=-\frac {1312} {n^2}\text { kJ/mol}. E n. 39.50. Model: Photons are emitted when an atom undergoes a quantum jump from a higher energy level to a lower energy level. On the other hand, photons are absorbed in a quantum jump from a lower energy level to a higher energy level. Because most of the atoms are in the n = 1 ground state, the only quantum jumps in the absorption spectrum start from the n = 1 state.
The figure shows an energy level diagram for the hydrogen atom. Several transitions are shown and are labeled by letters.Note: The diagram is not drawn to scale. Which transition corresponds to the absorption of the photon with the longest wavelength?
The x-axis shows the allowed energy levels of electrons in a hydrogen atom, numbered from 1 to 5. The y-axis shows each level's energy in electron volts (eV). One electron volt is the energy that an electron gains when it travels through a potential difference of one volt (1 eV = 1.6 x 10 -19 Joules).
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n
The energy level diagram is for a hypothetical atom. A gas of these atoms initially in the ground state is irradiated with photons having a continuous range of energies between 7 and 10 electron volts. One would expect photons of which of the following energies to be emitted from the gas? (A) 1, 2, and 3 eV only (B) 4, 5, and 9 eV only
Figure 5. An energy-level diagram plots energy vertically and is useful in visualizing the energy states of a system and the transitions between them. This diagram is for the hydrogen-atom electrons, showing a transition between two orbits having energies E 4 and E 2.
The greatest possible fall in energy will therefore produce the highest frequency line in the spectrum. The greatest fall will be from the infinity level to the 1-level. (The significance of the infinity level will be made clear later.) The next few diagrams are in two parts - with the energy levels at the top and the spectrum at the bottom.
We like representing these energy levels with an energy level diagram. The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Let's say our pretend atom has electron energy levels of zero eV, four eV, six eV, and seven eV.
An electron with 1.7 eV of kinetic energy collides with an atom whose energy-level diagram is shown in (Figure 1). The electron kicks the atom into an excited state. What is the electron's kinetic energy after the collision?
Solution for 24. I FIGURE EX38.24 is an energy-level diagram for a simple atom. What wavelengths, in nm, appear in the atom's (a) emission spectrum and (b)…
An energy-level diagram for a hydrogen atom and several possible atomic transitions are shown in Figure 5.20. When we measure the energies involved as the atom jumps between levels, we find that the transitions to or from the ground state, called the Lyman series of lines, result in the emission or absorption of ultraviolet photons.
The figure is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum? #1: From wavelength 3 to 1. #2: From wavelength 3 to 2. #3: From wavelength 2 to 1. What wavelengths appear in the atom's absorption spectrum?
Calculate the wavelength associated with the following energy transitions: (d) n = 3 to n = 2 (e) n = 2 to n = 1 (f) n = 3 to n = 1 _____ 2) The figure above shows the energy-level diagram for a hypothetical simple atom. The wavelength of the radiation emitted when an electron undergoes transition B is 400 nm (or 400 x 10-9 m), and for ...
Visualising energy levels . In an atom, electrons around a central nucleus can only have particular energy values. These discrete values are termed 'energy levels'. In a diagram they are represented by horizontal lines, with the lowest level (the ground state) at the bottom and the highest level (ionisation) at the top. The levels are a consequence of the wave nature of electrons, as described ...
4) The figure shows part of the energy level diagram of a certain atom. The energy spacing between levels 1 and 2 is twice that between 2 and 3. If an electron makes a transition from level 3 to level 2, the radiation of wavelength λ is emitted.



![Left: energy diagram of atomic oxygen [28] and radiative ...](https://www.researchgate.net/profile/Marcel-Fiebrandt/publication/339736227/figure/fig2/AS:865904845918208@1583459211746/Left-energy-diagram-of-atomic-oxygen-28-and-radiative-transitions-taken-into-account.png)



















0 Response to "35 the figure is an energy-level diagram for a simple atom. (figure 1)"
Post a Comment