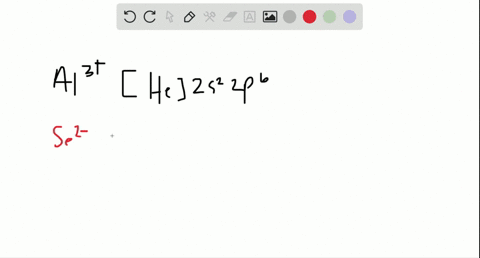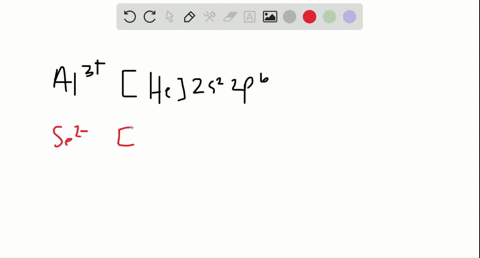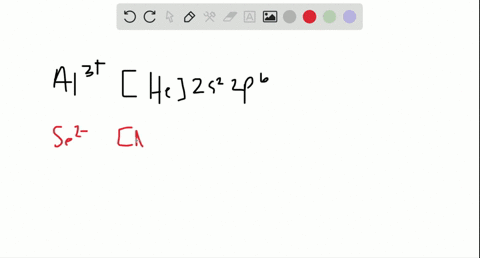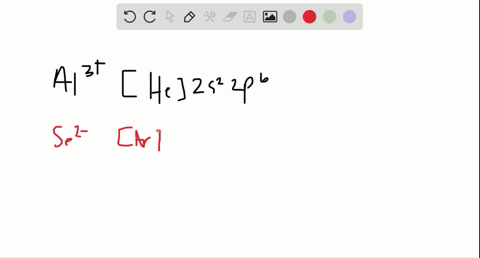
35 enter the orbital diagram for the ion mo3+.
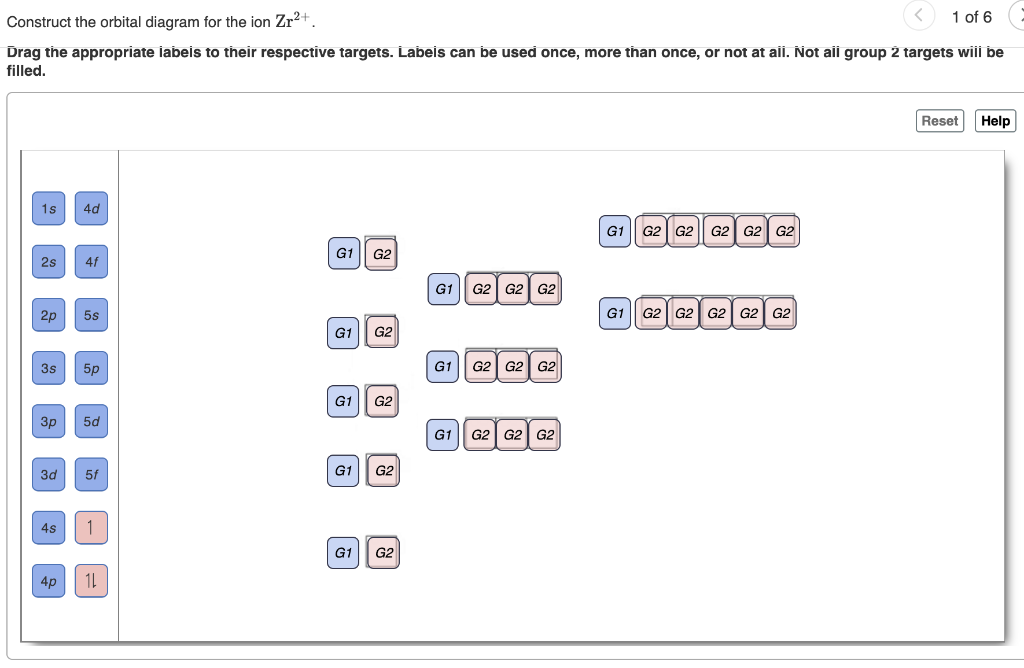
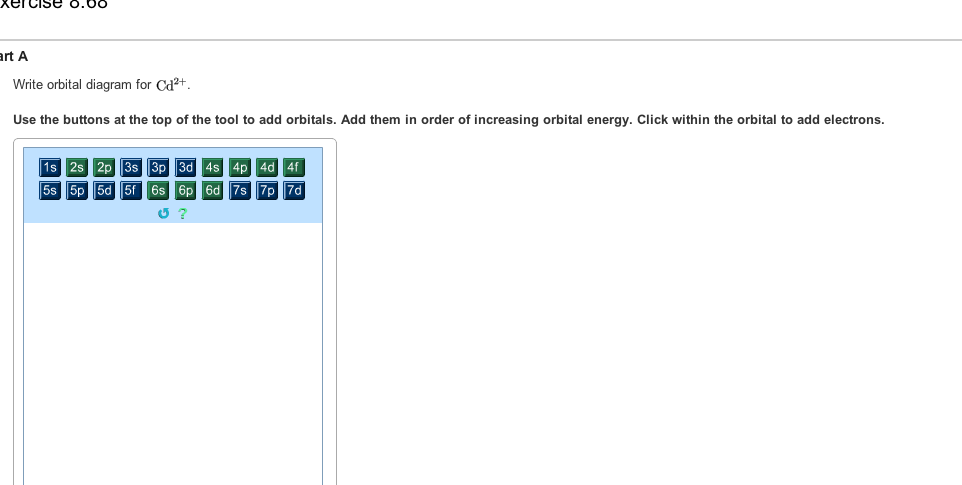
Mo3+ Orbital Diagram. Compact version of orbital energy diagram with each orbital represented . Mo2+, Mo3+, Mo4+ and Mo5+ are all known in aqueous solution. That's why Mo3+ has 39 electrons instead of 42 in its ground state electron The normal electron configuration of zinc is [Ar] 3d 10 4s 2, with 2 4s orbital. Answer to Write orbital diagram for Mo3+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing.write orbital diagram for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd 2+ diagramweb.net + diagramweb.net 3+ d. Zr 2+ Provide your answer: example:paramagnetic, diamagnetic, etc., .
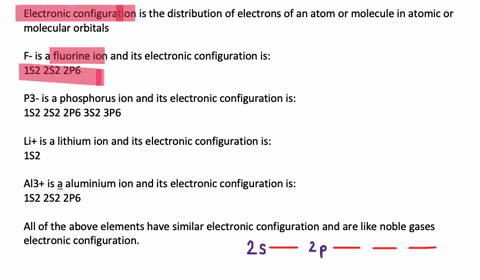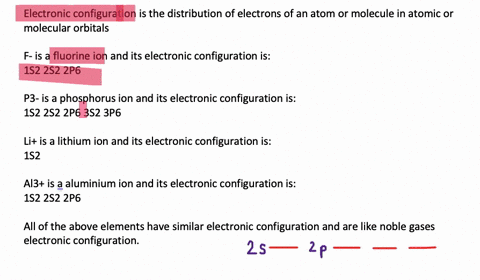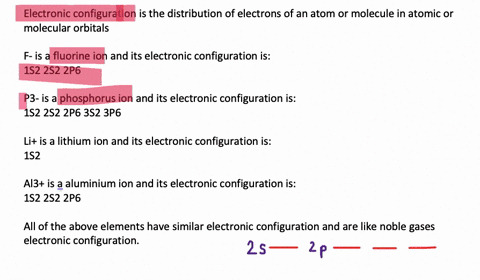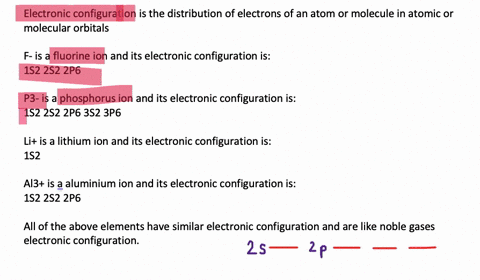
Electron Configuration, [Kr] 4d5 5s1. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d5 5s1. Orbital Diagram. 1s. You should keep in mind that the 5s orbital gets filled before the 4d orbital, however, when removing electrons; the electrons will be removed. Write the condensed ground-stste electron configuration of the transition metal ion Mo3+ ..

Enter the orbital diagram for the ion mo3+.
1 answerThe symbol Mo is used to represent a chemical element molybdenum. The atomic number of Mo is 42. Its electron configuration is {eq}\left[... 3 Nov 2015 · 1 answerSee explanation. Explanation: The electron configuration of 15P is: 15P:1s22s22p63s23p3. When phosphorous gains 3 electrons to form the ion ... Enter the orbital diagram for the ion Mo3+ ‣ When an element is a cation (+) you REMOVE electrons. ‣ Electrons are generally removed from the "s" sub-level 1.) Remove 2 electrons from 5s2 2.) Remove 1 electron from 4d4 ANSWER: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4d3.
Enter the orbital diagram for the ion mo3+.. The electron added would go into the 3pz orbital. b) The orbital diagram shows the 2s and 2p orbitals filled which would represent the last element in period 2, Ne. .. a) Mo3+: [Kr]4d 3. The electron gained would go into the 2pz orbital and is the second electron in that Y 4d a) The orbital diagram shows the element is in period 4 (n = 4 as .. Write orbital diagram for Mo3+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within. Write the condensed ground-stste electron configuration of the transition metal ion Mo3+ .. (16 points) Write the electron configuration for H. (1 point) Write the electron configuration for O. Enter the orbital diagram for the ion mo3. Ar3d 5 d hf 2. Enter the orbital diagram for the ion mo3. Enter the orbital diagram for the ion mo 3. Used in electronics jewelry and coins. Add them in order of increasing orbital energy. Click within the orbital to add electrons. Click within the orbital to add electrons. Write orbital diagram for au. Orbital diagram for au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 you just have to fill the boxes with arrows s orbital has only one box with two arrows. Enter the orbital diagram for the ion mo3. Enter the orbital diagram for the ion zr2. Use the buttons at the top of the tool to a. Enter the orbital diagram for the ion mo 3.
This means that it is easier for the electron in the 5s orbital to leave. So, the 5s electron get ionized first. After the 5s electron leave, the next two electrons to be ionized comes from the 4d orbital. Therefore, the electronic configuration of Mo3+ is. [Kr]4d3. Question: Part Enter the orbital diagram for the ion Mo3+ Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital ... Mo is 42 on the periodic table, since the question asks for Mo3+, you have to subtract 3 electrons. That's why Mo3+ has 39 electrons instead of 42 in its ground ...1 answer · 0 votes: Mo: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d4 Mo 3+: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s0 4d3 ... To write the configuration for the Molybdenum and the Molybdenum ion, first we need to write the electron configuration for just Molybdenum (Mo). We first n...
Enter the orbital diagram for the ion Mo3+ ‣ When an element is a cation (+) you REMOVE electrons. ‣ Electrons are generally removed from the "s" sub-level 1.) Remove 2 electrons from 5s2 2.) Remove 1 electron from 4d4 ANSWER: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4d3. 3 Nov 2015 · 1 answerSee explanation. Explanation: The electron configuration of 15P is: 15P:1s22s22p63s23p3. When phosphorous gains 3 electrons to form the ion ... 1 answerThe symbol Mo is used to represent a chemical element molybdenum. The atomic number of Mo is 42. Its electron configuration is {eq}\left[...

Material Design Concept Of Lithium Excess Electrode Materials With Rocksalt Related Structures For Rechargeable Non Aqueous Batteries Yabuuchi 2019 The Chemical Record Wiley Online Library
Solved Write Orbital Diagrams For Each Ion And Determine If The Ion Is Diamagnetic Or Paramagnetic A Cd2 B Au C Mo3 D Zr2
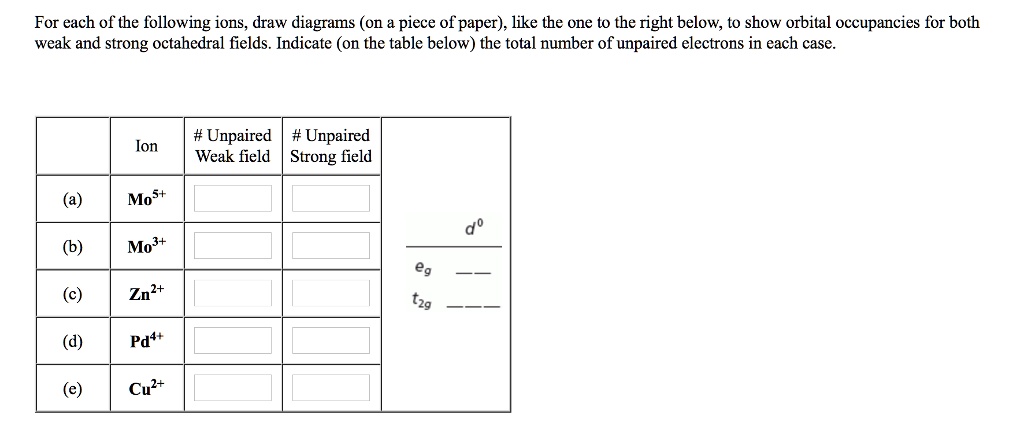
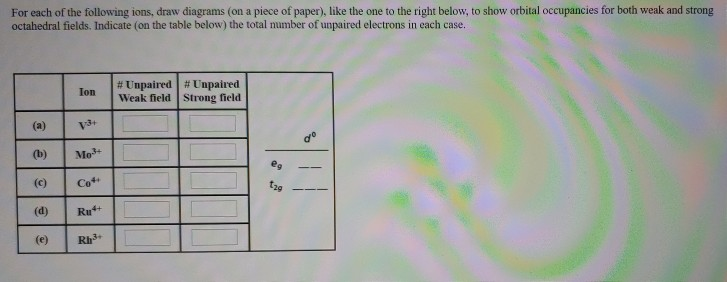
Solved For Each Of The Following Ions Draw Diagrams On Piece Of Paper Like The One To The Right Below To Show Orbital Occupancies For Both Weak And Strong Octahedral Fields Indicate On
Solved Write Orbital Diagrams For Each Ion And Determine If The Ion Is Diamagnetic Or Paramagnetic A Cd2 B Au C Mo3 D Zr2
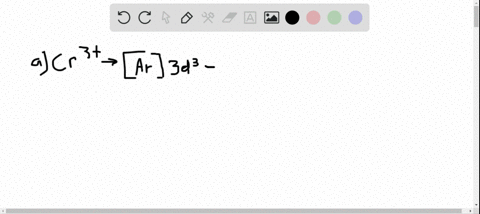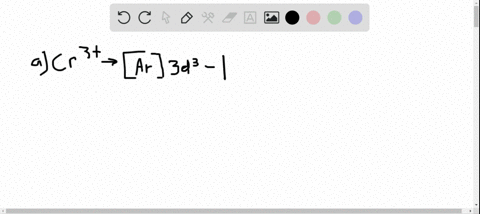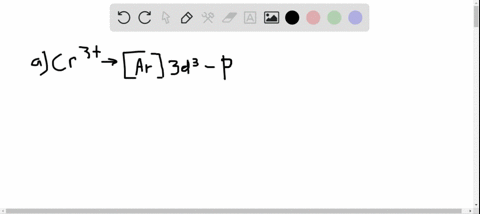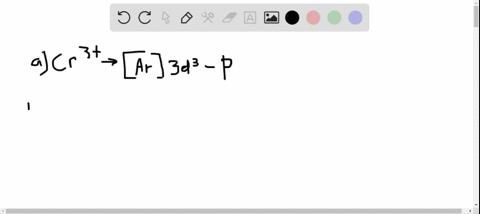
Solved Write Orbital Diagrams For Each Ion And Determine If The Ion Is Diamagnetic Or Paramagnetic A Cd2 B Au C Mo3 D Zr2















0 Response to "35 enter the orbital diagram for the ion mo3+."
Post a Comment