35 bohr diagram for gold
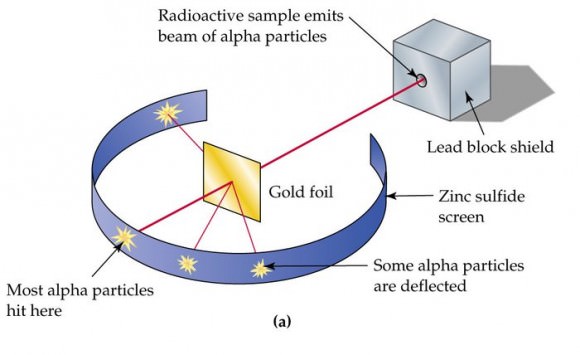
Remembering that the x-y diagram is a plot of vapor (V) vs. liquid (L), it can be seen that the operating line is a simple y=mx+b equation. Therefore, with the ... Equation, commonly known as the q-line. • q = molf i f d liidile fraction of saturated liquid in feed stream x=molefractionofanycomponentinx = mole fraction of any component in The gold foil experiment was a pathbreaking work conducted by scientists Hans Geiger and Ernest Marsden under the supervision of Nobel laureate physicist Ernest Rutherford that led to the discovery of the proper structure of an atom. Known as the Geiger-Marsden experiment, it was performed at the Physical Laboratories of the University of ...
Generalized energy-level diagram for atomic orbitals in an atom with two or more electrons (not to scale). Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first. Thus, many students find it confusing that, for example, the 5 p orbitals …

Bohr diagram for gold
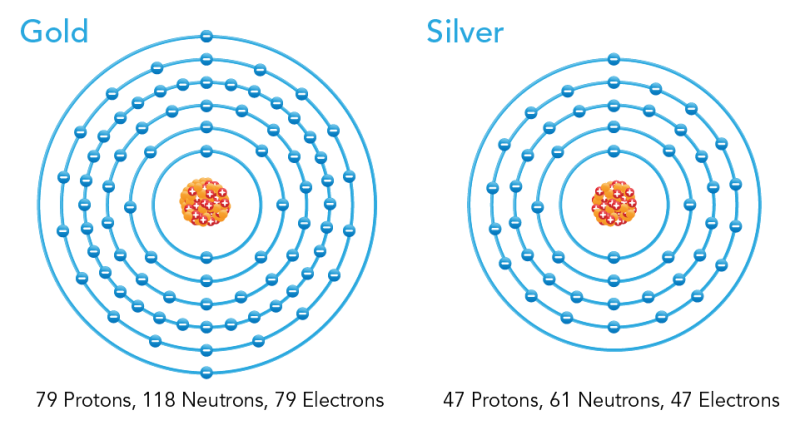
Gold has 79 protons and electrons inside each isotope. These isotopes also contain 118 neutrons. Gold only has one stable isotope, while there are 36 unstable ... Valance Electrons, Quantum Numbers and Bohr Model Diagram. Valance electrons are the electrons on the outermost ring. They can react with other elements during chemical reactions. Gold's dot diagram to the left contains one valance electron. The four quantum numbers for the last electron in gold's orbital diagram are 5,2, 2, -1/2. Answer: 1. Find the gold element on the periodic table. It's symbol is “Au” you'll find the gold's atomic number and atomic mass on the periodic table. 2. Determine the number of electrons. It's the same as the atomic number > 6 3. You need to notice the gold's row in thr periodic table. It's 6. ...
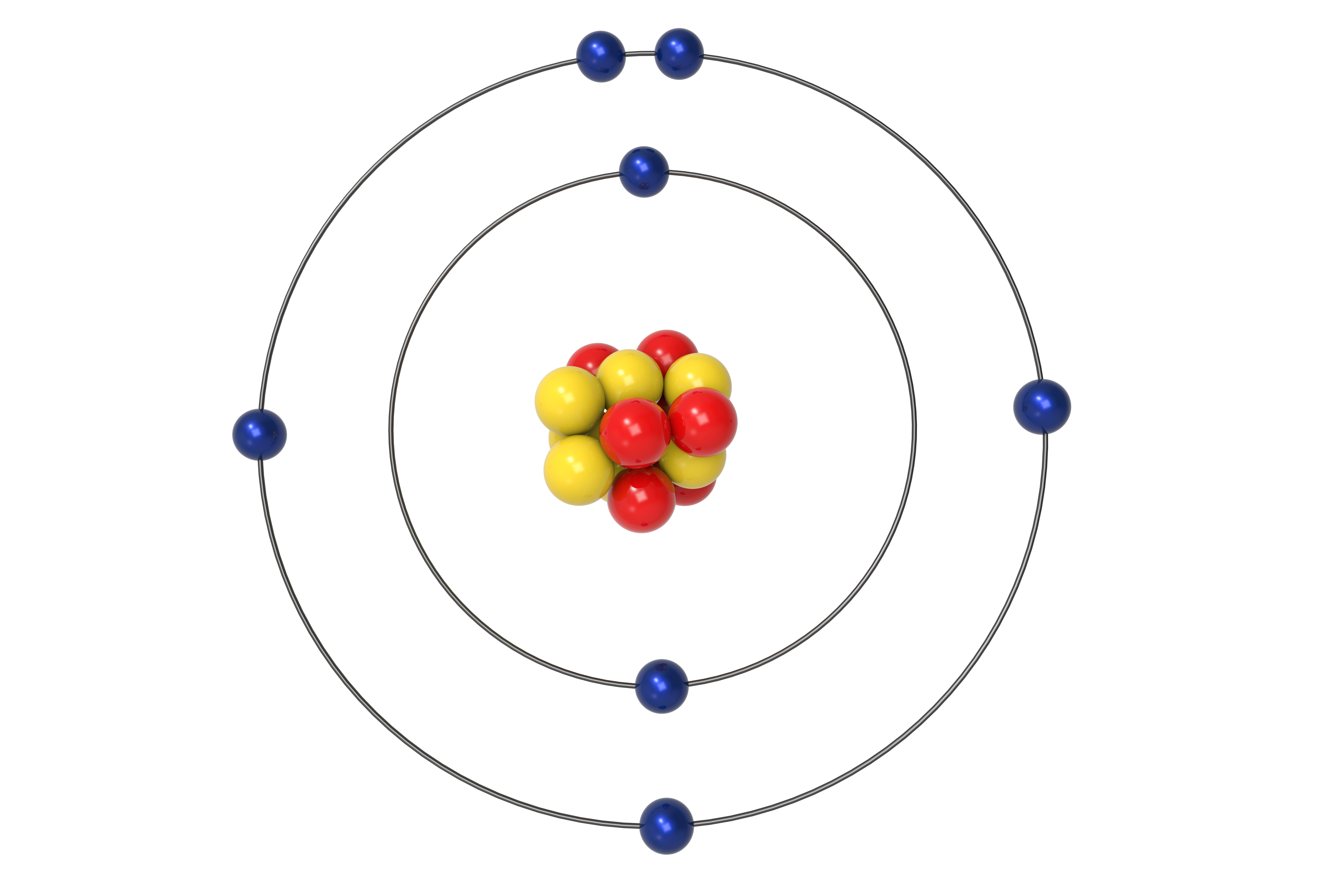
Bohr diagram for gold. Name: Gold Symbol: Au Atomic Number: 79 Atomic Mass: 196.96655 amu Melting Point: 1064.43 °C (1337.5801 K, 1947.9741 °F) Boiling Point: 2807.0 °C (3080.15 K, 5084.6 °F) Number of Protons/Electrons: 79 Number of Neutrons: 118 Classification: Transition Metal Crystal Structure: Cubic Density @ 293 K: 19.32 g/cm 3 Color: Gold Atomic Structure Gold is the most malleable of all metals and soft enough to be cut with a knife. Stone age peoples hammered gold into plates for ornamental purposes. Really quite large amounts were gathered together. Though King Tutankhamun was a minor Pharaoh and died aged 18, his coffin alone contained 112 kg of gold. Gold (Au). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of gold-197 (atomic number: 79), the most common isotope of this element. The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79 electrons (white) successively occupy available electron shells (rings). Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
26 Mar 2020 — The gold atomic structure has 79 protons and 118 neutrons in its nucleus. It has 79 electrons. These electrons will exist in determined ... Name: Gold Symbol: Au Atomic Number: 79 ... Number of Protons/Electrons: 79. Number of Neutrons: 118 ... [Bohr Model of Gold], Number of Energy Levels: 6. Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of Aluminum (Al) 2, 8, 3: 14: Bohr model of ... and Bohr's Model Q6. Expansion of Bohr's Model Q7.Wave Mechanics Q8. Tunneling----- S-6.The X-ray Sun S-7.The Sun's Energy S-7A. The Black Hole at our Galactic Center LS-7A. Discovery of Atoms and Nuclei S-8.Nuclear Power S-9.Nuclear Weapons: The Atomic Nucleus Planck's law described the way hot matter in bulk radiated energy. To gain ...
Gold Bohr Diagram by Emma Turlo - October 29, 2012. Chemistry videos to help you simplify your studying. Our videos prepare you to succeed in your college classes with concepts, examples, and practice problems. Answer: 1. Find the gold element on the periodic table. It's symbol is “Au” you'll find the gold's atomic number and atomic mass on the periodic table. 2. Determine the number of electrons. It's the same as the atomic number > 6 3. You need to notice the gold's row in thr periodic table. It's 6. ... Valance Electrons, Quantum Numbers and Bohr Model Diagram. Valance electrons are the electrons on the outermost ring. They can react with other elements during chemical reactions. Gold's dot diagram to the left contains one valance electron. The four quantum numbers for the last electron in gold's orbital diagram are 5,2, 2, -1/2.
Gold has 79 protons and electrons inside each isotope. These isotopes also contain 118 neutrons. Gold only has one stable isotope, while there are 36 unstable ...

Bohr Model Atom Electron Shell Copper Rutherford Model Elements Electron Elements Vector Rim Png Pngwing

Atomic Models This Model Of The Atom May Look Familiar To You This Is The Bohr Model In This Model The Nucleus Is Orbited By Electrons Which Are In Ppt Download

Bohr Model Electron Shell Atom Valence Electron Electron Configuration Periodic Table Of Elements Angle Symmetry Smiley Png Pngwing
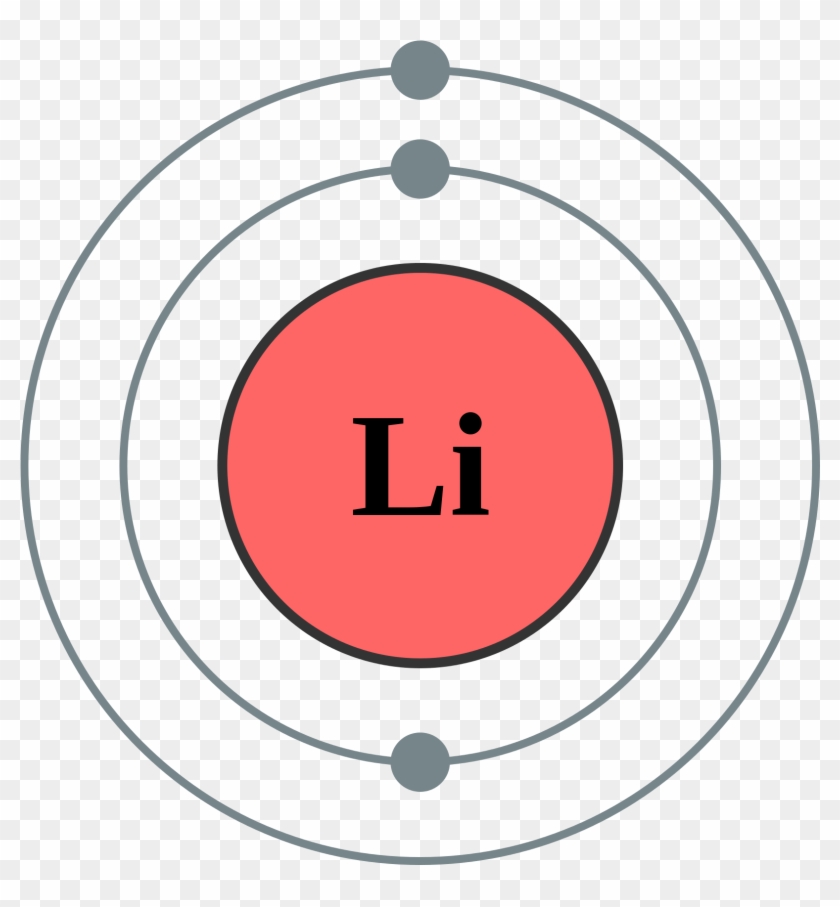
Diagram Bohr Diagram For Boron Rh Drdiagram Com Ion Modelo De Bohr Litio Free Transparent Png Clipart Images Download
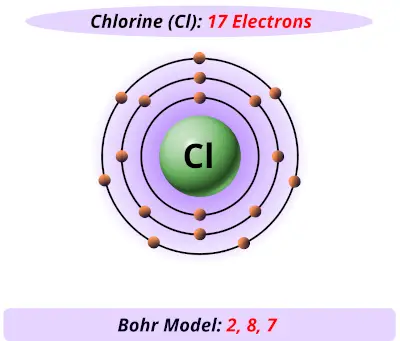


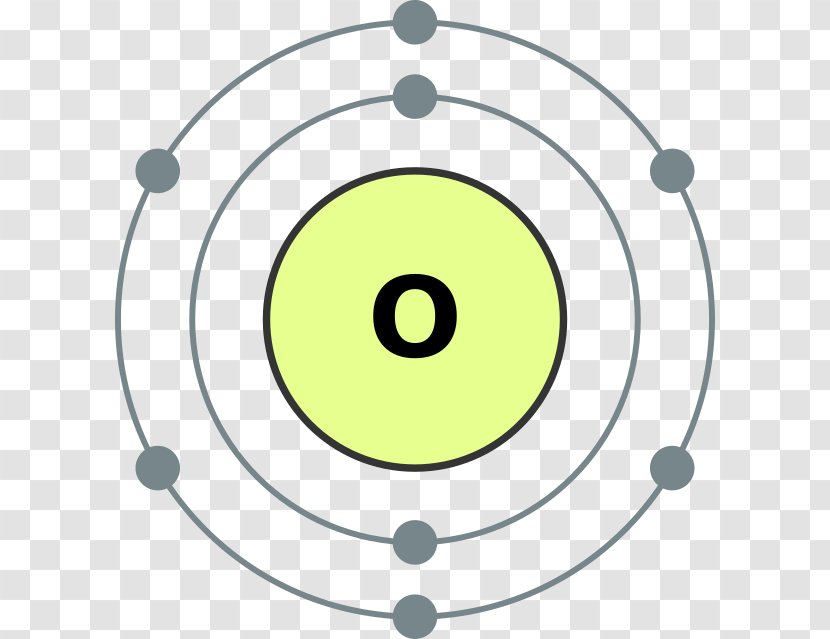



:max_bytes(150000):strip_icc()/Selenium-58b601fd3df78cdcd83d2a90.jpg)






.jpg)



0 Response to "35 bohr diagram for gold"
Post a Comment