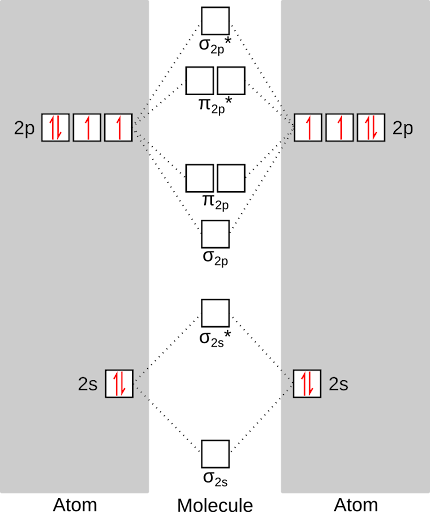
39 li2+ molecular orbital diagram
Li2- Molecular Orbital Diagram Molecular orbital energy level of Li2. diagram; the MOs go between them. Consider the MO diagram for Li2. This is a bond between two lithium atoms, which have an electron configuration of. The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital. Stability of the species Li2, Li2^- and Li2^+ increases in ... Click here👆to get an answer to your question ️ Stability of the species Li2, Li2^- and Li2^+ increases in the order: Solve Study Textbooks Guides. Join / Login >> Class 11 >> Chemistry >> Chemical Bonding and Molecular Structure >> Molecular Orbital Theory >> Stability of the species Li2, Li2^- and .
Molecular Orbital Theory - Chemistry Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds.

Li2+ molecular orbital diagram
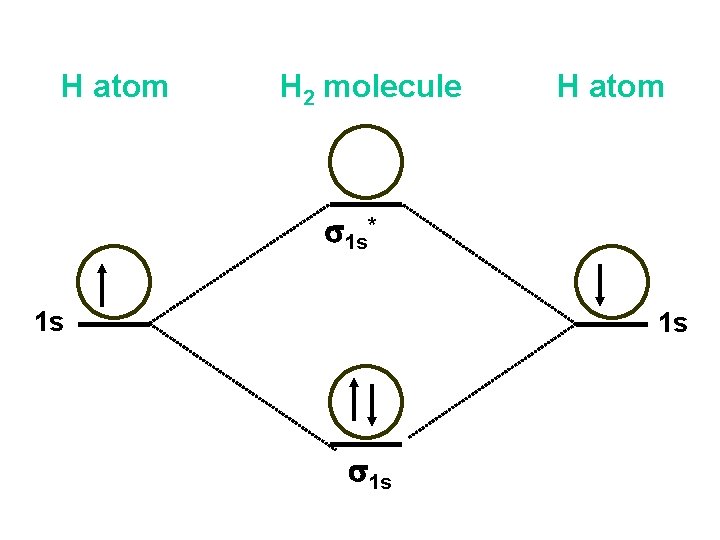
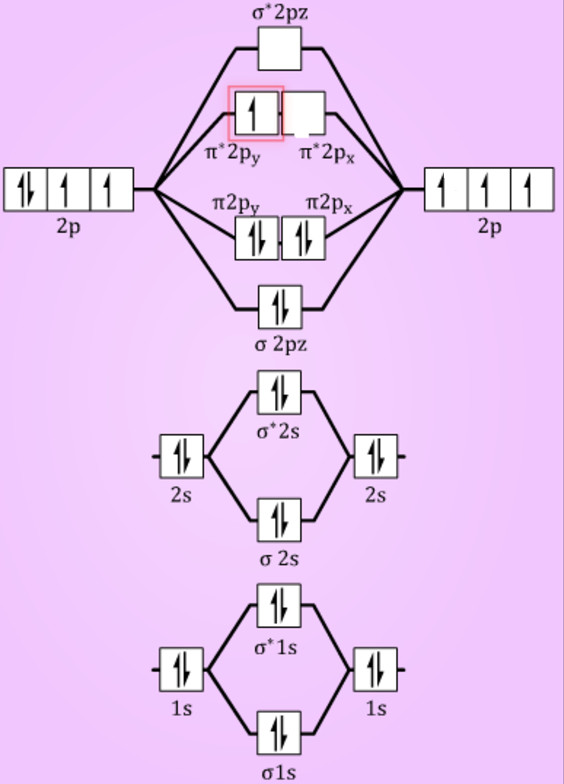
Molecular Orbitals - Introductory Chemistry - 1st Canadian ... The head-to-head overlap giving σ molecular orbitals results in greater overlap, making its bonding molecular orbital the most stable and lowest energy, while the σ* antibonding is least stable and has the highest energy (Figure 9.24 "Molecular orbital energy diagram for homonuclear diatomic molecules made from atoms of atomic number 8-10"). PDF Miessler-Fischer-Tarr5e SM Ch 05 CM molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold rotation axis. The 2p and 2p * orbitals exhibit Cs symmetry. The latter do not possess C2 rotation axes coincident to the Solved Construct the molecular orbital diagram for Li2. Note ... Chemistry. Chemistry questions and answers. Construct the molecular orbital diagram for Li2. Note that the 1s orbitals are not shown. Li Li Li Answer Bank 2s 2s、、 02s Identify the bond order O 0 O 05 O 1 O 1S 02.5. Question: Construct the molecular orbital diagram for Li2. Note that the 1s orbitals are not shown.
Li2+ molecular orbital diagram. PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in Write the electronic configuration of Lithium (Li2 ... Ground state electronic configuration of valence shell electrons in nitrogen molecule ( N 2 ) is written as ( S 2 s ) 2 ( S ∗ 2 s ) 2 ( P 2 p ) 4 ( S 2 p ) 2 Hence, the bond order of nitrogen molecule is: Medium. View solution. Solved Given the molecular orbital diagram for dilithium ... This problem has been solved! Given the molecular orbital diagram for dilithium (Li2) below, what would be the bond order of Li2+? This is the best answer based on feedback and ratings. No. of Bonding el …. View the full answer. Transcribed image text: Given the molecular orbital diagram for dilithium (Li2) below, what would be the bond order ... Draw MOT diagram for B2 molecule and calculate its class ... The molecular orbital diagram for B 2 molecule is as follows: We know that bond order is the difference between the number of bonds and the antibonds. Now, we have to calculate the bond order of B 2 molecule using the formula as follows: Bond order = 1 2 ( Number of electrons in BMO) − ( Number of electrons in ABMO) From the diagram, we can ...
Bonding in Homonuclear Diatomic Molecules: Li2, Li2+ ... Get access to the latest Bonding in Homonuclear Diatomic Molecules: Li2, Li2+, Be2, B2, C2, N2 prepared with IIT JEE course curated by undefined on Unacademy to prepare for the toughest competitive exam. How many valence electrons are in li2 The combination of two lithium atoms to form a lithium molecule, Li2, is analogous to the formation of H2, but the atomic orbitals involved are the valence 2s orbitals. Each of the two lithium atoms has one valence electron. Hence, we have two valence electrons available for the σ2s bonding molecular orbital. Molecular orbital : A molecule in which all the electrons ... Molecular orbital. source : chemed.chem.purdue.edu. Relationship between Electronic configuration and Molecular behaviour : 1) Bond order : It is defined as the number of covalent bonds between the two combining atoms of a molecule. Molecular Orbital Diagram Maker - University of Sydney ©2022 Prof Adam J Bridgeman | close window : ©2022 Prof Adam J Bridgeman | close windowProf Adam J Bridgeman | close window
Why "Li"_2^+ is more stable than "Li"_2 ... - Socratic.org Li2 is more stable than Li+ 2, because the bond is (hypothetically) stronger (probably gas-phase). Here we consider the molecular orbital diagram (MO) of Li2: The bond order can be calculated in a simple manner. Just take electrons that are in each MO, and Molecular Orbital Diagram (MO Diagram) of Li2 - YouTube Full first shell:sigma-1s and sigma1s* are both full.sigma-2s is full; sigma-2s* is empty.Bond order 1 = single bond.Check me out: (Solved) - molecular orbital diagram. li2 is stable but ... Nb= Number of electrons in bonding orbitals Na= Number of electrons in anti-bonding orbitals According to Molecular Orbital Theory If Nb > Na, the molecule is stable If Nb=Na, the molecule is unstable If Nb Use the molecular orbital diagram shown to determine which ... Keywords: MO diagram, C22+, B22-, Li2-, molecular orbital diagram, paramagnetic, diamagnetic, paramagnetism, diamagnetism, paired electrons, unpaired electrons. Answer : For a species to be diamagnetic it needs to have no unpaired electrons and for paramagnetic the species needs t have unpaired electrons.
Arrange the following in order of decreasing stability. a ... Keywords: MO diagram, C22+, B22-, Li2-, molecular orbital diagram, paramagnetic, diamagnetic, paramagnetism, diamagnetism, paired electrons, unpaired electrons. Answer : For a species to be diamagnetic it needs to have no unpaired electrons and for paramagnetic the species needs t have unpaired electrons.
Li2 Molecular Orbital Diagram Molecular orbital energy level of Li2.This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as .
Which has a greater bond length, Li2+ or Li2-? - Quora Molecular orbital electronic configuration of Li₂- : σ1s² σ*1s²σ2s2 σ*2s¹, bond order = (Nb - Na)/2 = (4-3)/2 = 0.5. As the bond orders are same in both the species, it is expected that their bond lengths are same. But as more electrons in anti bonding orbital lengthens the bond, the bond length of Li₂- is slightly greater than that of Li₂+.
Li2- Molecular Orbital Diagram - schematron.org Dec 03, 2019 · The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital. The molecule . Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = (). B.O = 1.
Energy level diagram for Molecular orbitals - Chemical ... Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ...
MOT | Molecular Orbital Energy level Diagram for Li2, Li2 ... This chemistry video tutorial provides a basic introduction into molecular orbital theory. It describes the formation of bonding and antibonding molecular o...
Is the bond in the molecular orbital energy level diagram ... A blank molecular orbital diagram (Part B 1 figure) has been provided to help you. Drag the formulas to the appropriate magnetic bin :C2^2+,Li2-,B2^2- Orbital question
40 c2 2 molecular orbital diagram - Wiring Diagram Images Li2- Molecular Orbital Diagram Consider the MO diagram for Li2. This is a bond between two lithium atoms, which have an electron configuration of. The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital. The molecule .Molecular orbitals of Li 2, Be 2 ...
Molecular orbital theory - W3schools Molecular orbital theory : As long as the specific molecular orbitals forms (their dependency on R and Z in the cylindrical coordination system) vary for each and every molecule, their dependency on an angle 'f' due to the represented by quantum number L and their behavior of G or U w.r.t to inversion are entirely determined by system's geometry.
Molecular Orbital Diagram For Li2 - schematron.org Oct 17, 2018 · Learn to draw molecular orbital electron configuration energy diagrams. molecular orbital electron configuration diagram for Li2 (Figure “Molecular orbital. This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation.
Molecular Orbital Diagram For Li2 - Wiring Diagrams Molecular orbital energy level of Li2. MO diagrams for Diatomic Molcules. Overview. In this section, we will compare MO diagrams for diatomic molecules X-X, from Li2 to Ne2. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = ().
Solved Construct the molecular orbital diagram for Li2. Note ... Chemistry. Chemistry questions and answers. Construct the molecular orbital diagram for Li2. Note that the 1s orbitals are not shown. Li Li Li Answer Bank 2s 2s、、 02s Identify the bond order O 0 O 05 O 1 O 1S 02.5. Question: Construct the molecular orbital diagram for Li2. Note that the 1s orbitals are not shown.
PDF Miessler-Fischer-Tarr5e SM Ch 05 CM molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold rotation axis. The 2p and 2p * orbitals exhibit Cs symmetry. The latter do not possess C2 rotation axes coincident to the
Molecular Orbitals - Introductory Chemistry - 1st Canadian ... The head-to-head overlap giving σ molecular orbitals results in greater overlap, making its bonding molecular orbital the most stable and lowest energy, while the σ* antibonding is least stable and has the highest energy (Figure 9.24 "Molecular orbital energy diagram for homonuclear diatomic molecules made from atoms of atomic number 8-10").
















![Solved] Using Figures 9.35 and 9.43 as guides, draw the ...](https://s3.amazonaws.com/si.question.images/image/images11/876-(557)-2.png)




0 Response to "39 li2+ molecular orbital diagram"
Post a Comment