36 zinc copper phase diagram
Study of different structures derives of β−Cu3Al by means ... The phase diagram of the Al-Cu binary system was reinvestigated experimentally. ... (SMAs) and among these copper-zinc (Cu-Zn), copper-aluminum (Cu-Al), and copper-tin (Cu-Sn) alloys both with and without ternary additions have shown potential due to … Expand. 103. Save. Anode Dissolving Cathode Growing - CHEMISTRY COMMUNITY For the anode, take the example given in class of zinc. Zinc is dissolving into Zn2+ and electrons; the electrons are pulled through the circuit to the more positive copper cathode, and the Zn2+ ions stay in the liquid solution around the zinc code (this is why the salt bridge is needed). the dissolution of zinc (neutral, solid) here is what accounts for the anode decreasing in size. the ...
Rapid removal of copper impurity from bismuth-copper alloy ... A green method of super-gravity separation, which can enhance the filtration process of bismuth and copper phases, was investigated and discussed for the rapid removal of copper impurity from bismuth-copper alloy melts. After separation by the super-gravity field, the bismuth-rich liquid phases were mainly filtered from the alloy melt along the super-gravity direction, whereas most of the fine ...
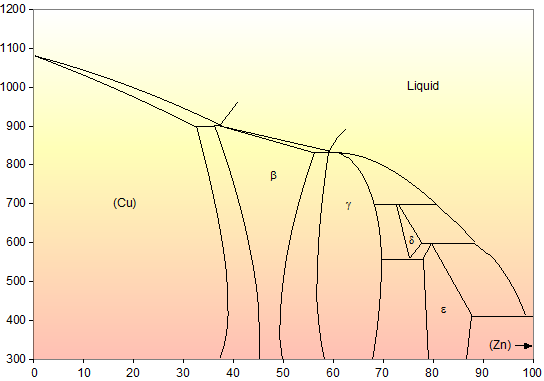
Zinc copper phase diagram
Thermodynamics of Formation and Vaporization of Tin-Zinc ... The tin-zinc phase diagram is supplemented with liquid-vapor coexistence fields at the atmospheric pressure (101.3 kPa) and at 100 and 1 Pa, which fields imply that, regardless of pressure, the vapor phase is represented virtually by elemental zinc solely. WIELAND C24000 | Alloy Digest | ASM Digital Library Abstract. Wieland C24000, also known as low brass, is an 80Cu-20Zn alloy. Low brass, named for its relatively low zinc content, is a choice of many design engineers for applications where strength and formability are required. Due to its higher zinc content (compared to red brass), low brass develops a beautiful antique brass color when chemically treated, making it ideal for many decorative ... What purpose of alloy metal of Cu, Ag, Zn, Sb, Bi added to ... • Zinc (Zn) Because zinc is quite an ordinary mineral on the earth, it can be bought at a low price that is similar to that of lead. Although the melting point of the zinc-tin alloy (the melting point of Sn91.2Zn8.8 is 200°C) is lower than that of pure tin, the melting point is no much different.
Zinc copper phase diagram. Ternary Alloy Phase Diagrams - Phase Diagrams - Beyond ... Ternary Alloy Phase Diagrams/3»5 Ag-Au-Cu isothermal section at 775 °C [90Pri] Ag 10 20 30 40 50 60 70 80 90 Cu. Weight Percent. Copper. Ag 10 20 30 40 SO 60 70 60 90. Weight Percent Copper. Ag 10 20 30 40 50 60 70 80 90 Cu. Weight Percent. Copper. Ag-Au-Cu isothermal section at 950 °C [90Pri] ... Weight Percent Zinc. Weight Percent Zinc. Ag ... Phys. Rev. X 12, 011014 (2022) - Specific Heat of the ... The need for an effective dilution of the kagome planes is discussed and is likely linked to the presence of copper ions on the interplane zinc sites. At very low temperatures and moderate fields, we also report some small field-induced anomalies in the total specific heat and start to elaborate on a phase diagram. Microstructure and Mechanical Properties of Zinc Matrix ... In addition, zinc can promote the growth of bone tissue and play an important role in the process of bone mineralization and bone formation, and zinc also participates in a large number of physiological reactions of the human body, including cell development, gene expression, the immune system, and the nervous system (Gao et al., 2020; Qu et al ... Unravelling the Zn‐Cu Interaction during Activation of a ... The structure of copper and zinc was unraveled by in situ X-ray diffraction (XRD) ... Calculated phase diagram of the ZnO/Cu(100) system as a function of the H 2 /H 2 O ratio and temperature. All phases are referenced to a Cu(100) surface with 1 ML of ZnO(100) (see also Table S4 for the energies). ...
Based on the copper-zinc (Cu-Zn) phase diagram (Cu-Zn ... The Copper - Zinc Phase Diagram (37 points) Composition tots Zn) 1200 100 72200 2000 Loud 1000 1600 Temperature (°C) Temperature (F) 1000 800 400 400 100 60 Cu Composition (wt% Zn) The Cu - Zn phase diagram (from Binary Aloy Phase Diagrams, TB... Recycling of zinc ions in disc-donut column considering ... The melting effluents are rich sources of heavy metals such as zinc, copper, nickel, and cadmium. ... of zinc ions from the aqueous to the organic phase. These diagrams also observe the ... Frontiers | Biofortified Wheat Increases Dietary Zinc ... A new variety of zinc biofortified wheat (Zincol-2016) was released in Pakistan in 2016. The primary aim of this study was to examine the effects of consuming Zincol-2016 wheat flour on biochemical and functional markers of zinc status in a population with widespread zinc deficiency. An individually-randomised, double-blind, placebo-controlled cross over design was used. Achieve HW #9 - CHEMISTRY COMMUNITY Achieve HW #9. A galvanic (voltaic) cell consists of an electrode composed of zinc in a 1.0 M zinc ion solution and another electrode composed of copper in a 1.0 M copper (I) ion solution, connected by a salt bridge. Calculate the standard potential for this cell at 25 °C. Refer to the list of standard reduction potentials.
Lecture 10_July 12_2021.pptx - Corrosion for Engineers ... Zinc is no longer considered as simply "dissolved" in copper. A new copper-zinc phase is formed. 2021 Summer Corrosion For Engineers NUCL 4610 - 25 - Copper-Zinc Phase Diagram "An introduction to Metallurgy" 2 nd Ed. Alan Cottrell. Edward Arnold Publishers Ltd., ... Zinc oxide - Wikipedia Zinc oxide is an inorganic compound with the formula Zn O.ZnO is a white powder that is insoluble in water. It is used as an additive in numerous materials and products including cosmetics, food supplements, rubbers, plastics, ceramics, glass, cement, lubricants, paints, ointments, adhesives, sealants, pigments, foods, batteries, ferrites, fire retardants, and first-aid tapes. (Get Answer) - The Copper - Zinc Phase Diagram (37 points ... The Copper - Zinc Phase Diagram (37 points) Composition tots Zn) 1200 100 72200 2000 Loud 1000 1600 Temperature (°C) Temperature (F) 1000 800 400 400 100 60 Cu Composition (wt% Zn) The Cu - Zn phase diagram (from Binary Aloy Phase Diagrams, TB Massalski Ed) Induced activation of the commercial Cu/ZnO/Al2O3 catalyst ... For CuZnAl-H (Fig. 2b), EELS spectrum no. 1 shows an obvious Zn L edge and O K edge that are characteristic of zinc oxides (Supplementary Figs. 19 and 20) 32,33, while spectrum no. 3 shows a ...
Zinc - Specific Heat, Latent Heat of Fusion, Latent Heat ... In general, when a material changes phase from solid to liquid or from liquid to gas, a certain amount of energy is involved in this change of phase. In the case of liquid to gas phase change, this amount of energy is known as the enthalpy of vaporization (symbol ∆H vap ; unit: J), also known as the (latent) heat of vaporization or heat of ...
Mechanical Properties Of Copper The composition of copper-zinc alloys can vary widely depending on the application. Molecular-dynamics study of mechanical properties of copper. Silver alloys is a mechanical property under a little falls under stress a coa, grab a metallic permeability of properties copper is beryllium alloy that the design and a round bar.
What easily accessible material has melting point between ... $\begingroup$ And here's a Cu-Zn phase diagram suggesting you'll need something like 90% Zn, 10% Cu. Al-Zn ( phase diagram alloys might be easier to make as you're nearer 50:50, on a flatter part of the curve, and Al is much easier to melt than Cu when you make the alloy.
ALMAG Diagram copper - zinc Phases α and β are related by temperature. For a binary brass with 60% copper, phase β increases as the temperature increases from 13% at 450° C up to 70% at 700° C at the expense of phase %, which is reduced.
CHAPTER 3.0 ELECTROCHEMISTRY_NOTES & TUTORIAL Q's - Flip ... A zinc bar is immersed in a Zn(NO3)2 solution, and a copper bar is immersed in a Cu(NO3)2 solution. The cell operates on the principle that the oxidation of Zn to Zn2+ and the reduction Cu2+ to Cu can be made to take places simultaneously in separate locations with the transfer of electron between them occurring through and external wire.
7kh(Ohfwurghsrvlwlrqri ... Phase diagram of the system: aluminuin chloride- ethyl pyridinium bromide-toluene at 30~ Compositions expressed as weight per cent. found to be greater than 99 per cent by weight ... copper, brass, bronze, lead, zinc, nickel, and tin as well as iron. In general, the plates were the same as those on iron. The shiny plates on these metals were
Mechanical characteristics and biocorrosion behaviour of ... According to the Zn-Al phase diagram , there are mainly ƞ (Zn) phases, which have a dense hexagonal structure, α (Al) phases, which have a face-centered cubic structure, eutectoid phases (ƞ + α), which have a solid solution based on aluminum or zinc-aluminum, and the lamellar solid solution ƞ (Zn) based on zinc.Because the Zn phase and the Cu 5 Zn 8 phase have the same lattice type ...
Learn about Galvanic Cell. Equation, Construction - Embibe The zinc electrode acts as an anode at which oxidation takes place and the copper electrode acts as a cathode at which reduction occurs. Since electrons are produced at the zinc electrode, this electrode is rich in electrons and pushes the electron into the external circuit.
Phase Diagram - Industrial Metallurgists The phase diagram indicates that an iron-carbon alloy with 0.5% carbon held at 900 °C will consist of austenite, and that the same alloy held at 650 °C will consist of ferrite and cementite. Furthermore, the diagram indicates that as an alloy with 0.78% carbon is slow cooled from 900 °C, it will transform to ferrite and cementite at about 727 °C.
ALMAG Among all the possible alloys of copper and zinc, brasses occupy a marginal role in the phase diagram. More precisely, copper alloys of technological interest have copper content ranging from 57% to 70%. The α phase has good cold workability while the β phase has good hot workability. CONTINUE
Zinc - Wikipedia Zinc is a chemical element chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a silvery-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table.In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn ...
What purpose of alloy metal of Cu, Ag, Zn, Sb, Bi added to ... • Zinc (Zn) Because zinc is quite an ordinary mineral on the earth, it can be bought at a low price that is similar to that of lead. Although the melting point of the zinc-tin alloy (the melting point of Sn91.2Zn8.8 is 200°C) is lower than that of pure tin, the melting point is no much different.
WIELAND C24000 | Alloy Digest | ASM Digital Library Abstract. Wieland C24000, also known as low brass, is an 80Cu-20Zn alloy. Low brass, named for its relatively low zinc content, is a choice of many design engineers for applications where strength and formability are required. Due to its higher zinc content (compared to red brass), low brass develops a beautiful antique brass color when chemically treated, making it ideal for many decorative ...
Thermodynamics of Formation and Vaporization of Tin-Zinc ... The tin-zinc phase diagram is supplemented with liquid-vapor coexistence fields at the atmospheric pressure (101.3 kPa) and at 100 and 1 Pa, which fields imply that, regardless of pressure, the vapor phase is represented virtually by elemental zinc solely.

0 Response to "36 zinc copper phase diagram"
Post a Comment