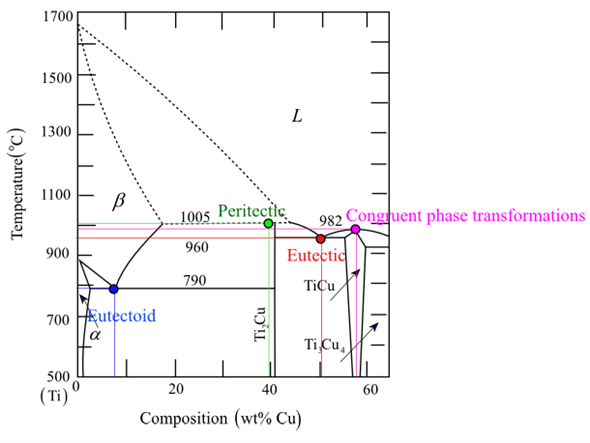
40 congruent transformation phase diagram
congruent transformations (congruent melting) transformations in which no change in composition for any phase is involved. e.g. allotropic transformations, melting of pure materials incongruent transformations (incongruent melting) the transformation involves a resulting phase that has a different composition than the phase(s) from which it ... Lection 4 Phase diagrams of unary and binary systems v Phase diagrams for unary systems, relations with the Gibbs energy, critical point; v Binary systems, relations with the Gibbs energy of phases, lever rule, types of phase diagrams: eutectic, peritectic, eutectoid, congruent transformation, monotectic etc. , relations with microstructure development Phase diagram is a chart showing ...
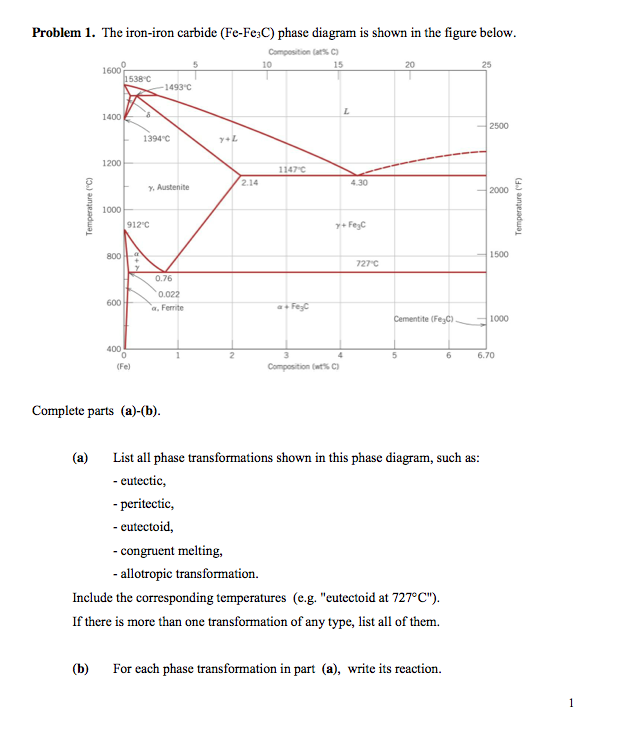
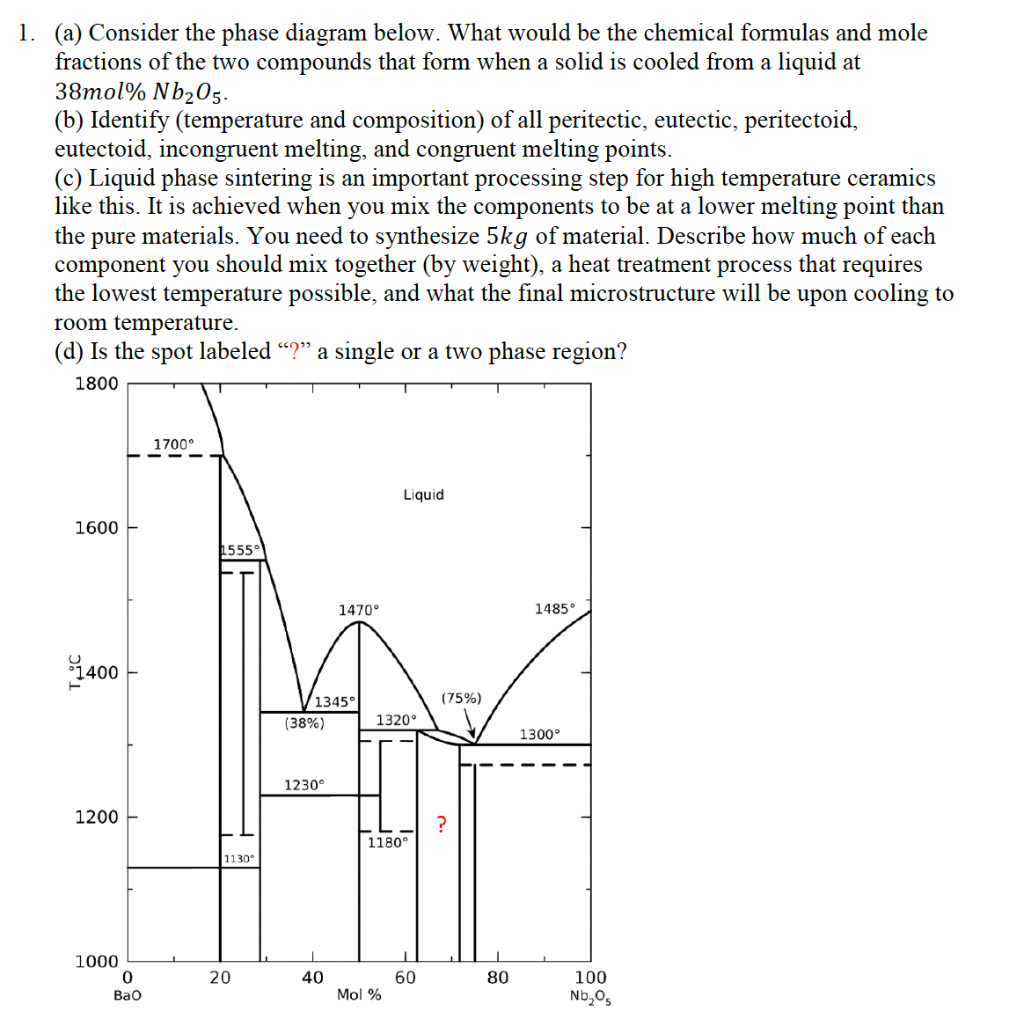
Figure 9.36 is the aluminum-neodymium phase diagram, for which only single-phase regions are labeled. Specify temperature-composition points at which all eutectics, eutectoids, peritectics, and congruent phase transformations occur. Also, for each, write the reaction upon cooling.
Congruent transformation phase diagram
For some given binary phase diagram upon heating or cooling, locate temperatures & compositions and write reactions of: Eutectic Eutectoid Peritectic congruent phase transformations 1/2/2015 1:48 PM Dr. Mohammad Abuhaiba, PE 4 Teach Yourself Phase Diagrams A.6 HRS 03/11/2009 and Phase Transformations DEF.The equilibrium constitution is the state of lowest Gibbs free energy G, for a given composition, temperature and pressure. An alloy in this state shows no tendency to change - it is thermodynamically A congruent phase transformation is "A transformation that the initial phase and the final phase have the same composition", no matter the initial phase is the solid phase or the liquid phase. What is the principal difference between congruent and incongruent phase transformation?
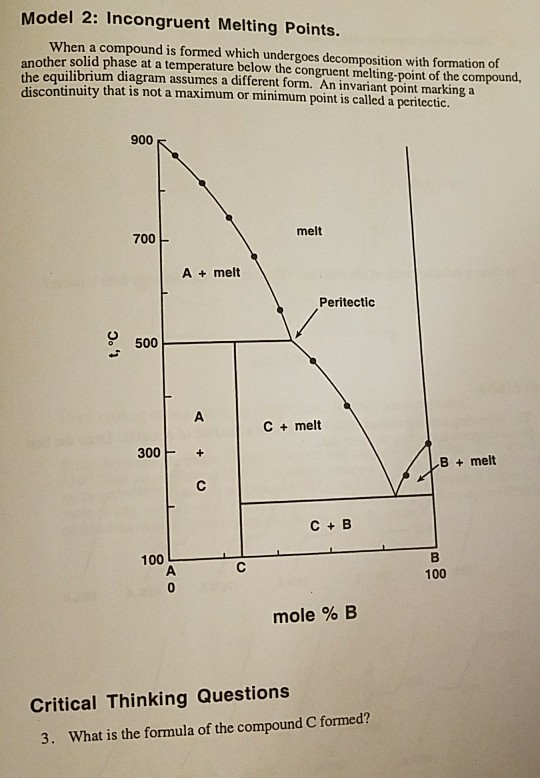
Congruent transformation phase diagram. A phase diagram is also called an equilibrium or constitutional diagram. It shows the relationship between temperature, the compositions and the quantities of phases present in an alloy system under equilibrium conditions. When temperature is altered many microstructure develop due to phase transformation. It may involve transition from one phase to another phase. Thus, these diagram are helpful in predicting phase transformation and the resulting microstructures. CONGRUENT PHASE TRANSFORMATIONS Congruent transformation: when there is no change in the composition for phases involved (Mg 2 Pb melts congruently) Incongruent transformation: change occurs in phase composition during transformation (peritectic reaction) An alloy of 20% Si is solidified in constant temperature of 363°C.Plot the phase diagram. (1) Label the phases (2) Draw the cooling curve an alloy of 50% Si and mark the micro structure change. 22. Two Metals Completely Soluble in the Liquid State but only Partly Soluble in the Solid State (Type III) 23. Alloy 1 24. Alloy 2 25. Alloy 3 26. Alloy 4 Intermetallic Compounds Monotectic Eutectoid & Peritectic Eutectoid and Peritectic Eutectoid & Peritectic Cu-Zn Phase diagram Congruent vs Incongruent Iron-Carbon System Iron Carbon Phase Diagram Cementite - What is it? Cementite has an orthorhombic lattice with approximate parameters 0.45165, 0.50837 and 0.67297 nm.
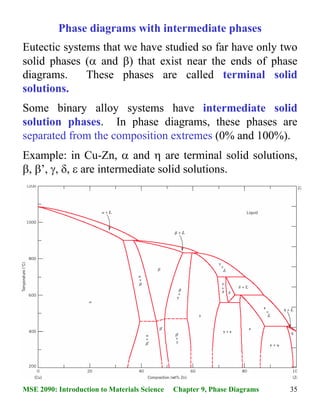
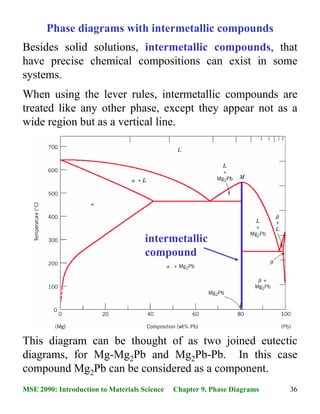
We will discuss different types of phase diagrams with intermediate phases crystallizing from the liquid state, and also analyze the phase diagrams with peritectoid transformation and ordering of intermediate phases in the solid state. Classification of intermediate phases. Phase diagrams with congruently melting compound 8:56 Taught By May 04, 2015 · 40MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams Congruent phase transformations A congruent transformation involves no change in composition (e.g., allotropic transformation such as α-Fe to γ-Fe or melting transitions in pure solids). For some given binary phase diagram upon heating or cooling, locate temperatures & compositions and write reactions of: Eutectic Eutectoid Peritectic congruent phase transformations 12/12/2015 11:23 AM Mohammad Suliman Abuhaiba, Ph.D., PE 4 Congruent, Incongruent transformations Phase transformations are two kinds -congruent and incongruent. Congruent transformation involves no compositional changes. It usually occurs at a temperature. E.g.: Allotropic transformations, melting of pure a substance. During incongruent transformations, at least one phase will
Appguy1 97 9 Add a comment 1 Answer Active Oldest Votes 2 A congruent phase transformation is "A transformation that the initial phase and the final phase have the same composition", no matter the initial phase is the solid phase or the liquid phase. source: Phase Diagrams in Metallurgy, written by Frederick N Rhines Share Improve this answer The congruent point on a phase diagram is where different phases of same composition are in equilibrium. The Gibbs-Konovalov Rule for congruent points, which was developed by Dmitry Konovalov from a thermodynamic expression given by J. Willard Gibbs, states that the slope of phase boundaries at congruent transformations must be zero (horizontal). of Cu in Sb. The b phase, which is a high-temperature phase, melts congruently at 683 C. It crystallizes in a cubic BiF 3-type structure (DO 3) with the space group Fm-3m. At the liquid melt, the Sb-rich b forms the g phase in a peritectic reaction (586 C). On the Cu-rich side, b and (Cu) are formed eutectically at 645 C. The b phase peritectics, and congruent phase transformations for the tin-gold system (Figure 9.36). There are two eutectics on this phase diagram. One exists at 10 wt% Au-90 wt% Sn and 217°C. The reaction upon cooling is L → α + β The other eutectic exists at 80 wt% Au-20 wt% Sn and 280°C. This reaction upon cooling is L → δ + ζ
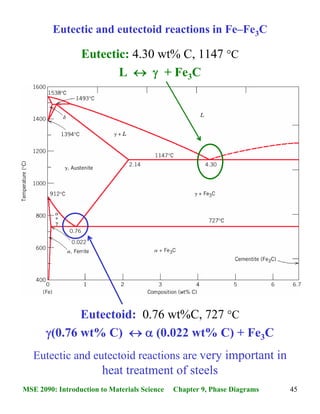
Congruent Phase Transformations A congruent transformation involves no change in composition (e.g., allotropic transformation such as a-Fe to g-Fe or melting transitions in pure solids). For an incongruent transformation, at least one phase changes composition (e.g. eutectic, eutectoid, peritectic reactions).
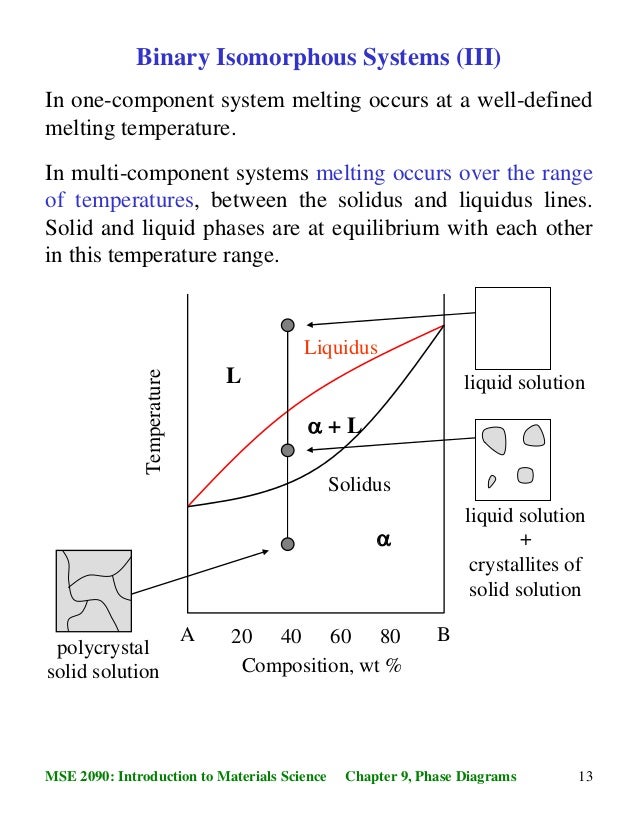
Fig. 2 (the liquid and solid solutions) are called conjugate phases. If the solidus and liquidus meet tangentially at some point, a maximum or minimum is produced in the two-phase field, splitting it into two portions, as shown in Fig. 3. Fig. 3 Schematic binary phase diagrams with solid state miscibility where the liquidus
Phase diagrams are used to map out the existence and conditions of various phases of a give system. The phase diagram of water is a common example. Water may stay in liquid, solid or gaseous states in ... congruent transformation and the latter incongruent transformations. Congruent transformations are cooling rate insensitive
Answer The principal difference between congruent and incongruent phase transformations is the occurrence of alterations on its composition during transformation.
In Mg-Pb phase diagram • three congruent melting points: pure Mg (1), pure Pb (2), and Mg 2 Pb (3) • All other transformations are incongruent Congruent transformation: composition does not change for all phases involved • Examples: melting of a pure material, melting of an intermetallic compound Incongruent transformation: composition ...
peritectics, and congruent phase transformations for a portion of the aluminum-copper phase diagram (Figure 9.37). There is one eutectic on this phase diagram, which exists at 8.3 wt% Al-91.7 wt% Cu and 1036°C. Its reaction upon cooling is L → α + β There are four eutectoids for this system. One exists at 11.8 wt% Al-88.2 wt% Cu and 565°C ...
Eutectic phase diagram for a silver-copper system. 2800 2600 2400 2200 2000 1800 1600 MgO CaO 20 40 60 80 100 0 C) L MgO ss + L MgO ss CaO ss + L CaO ss MgO ss + CaO ss Wt % Eutetic phase diagram for MgO-CaO system. Temperature (Lecture 19 - Binary phase diagrams 4 of 16 11/23/05
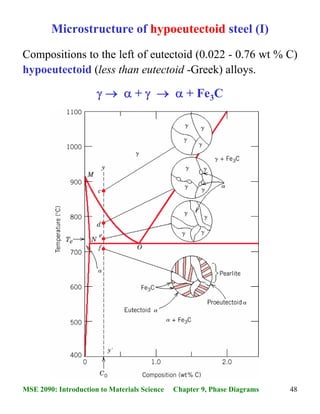
The end product of a congruent melting alloy shall have only one phase . The actual phase diagram may now be constructed by transferring the breaks on the cooling curves to a plot of temperature vs. composition, as shown in the figure given below.
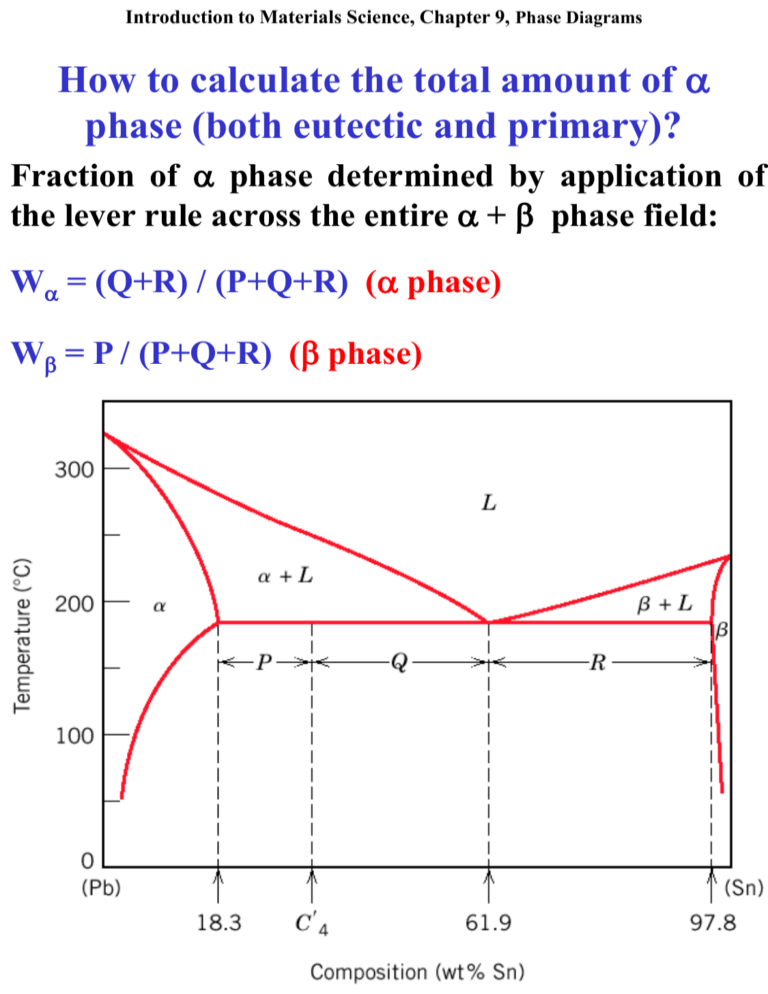
A and liquid (the only two phases present at this temperature) could be determined by measuring the distances a and b on figure 1. The percentages would then be given by the lever rule: % crystals of A = b/(a + b) x 100 % liquid = a/(a + b) x 100 Note that since the amount of crystals must increase with falling temperature the
Congruent melting point represents a definite temperature just like the melting points of pure components. In some phase diagrams, the congruent melting point of a compound AB may lie above the melting points of pure components A and B. But it is not always necessarily the case.
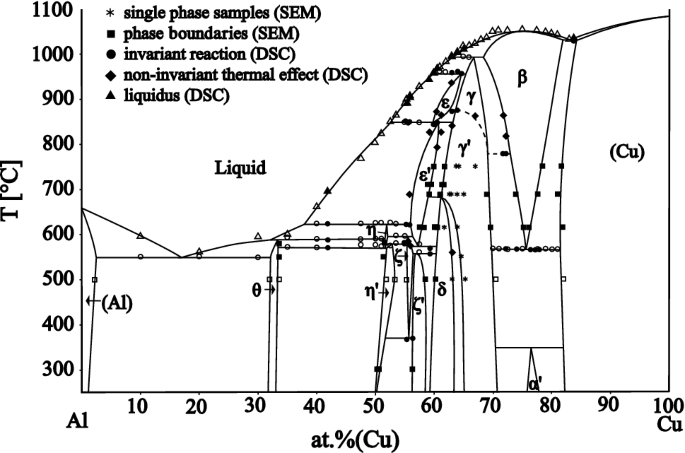
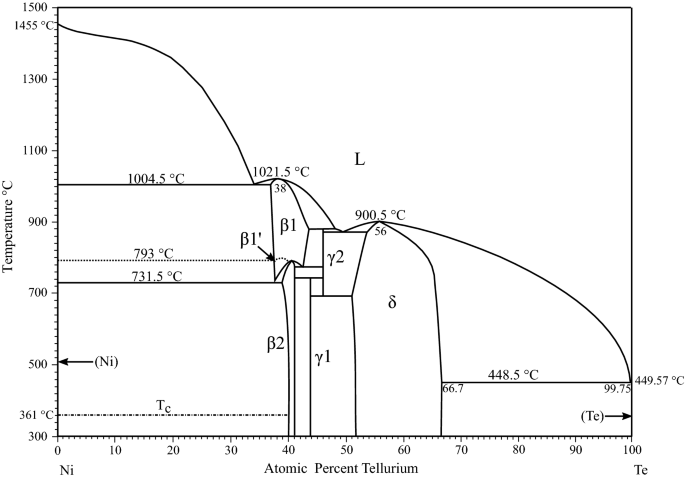
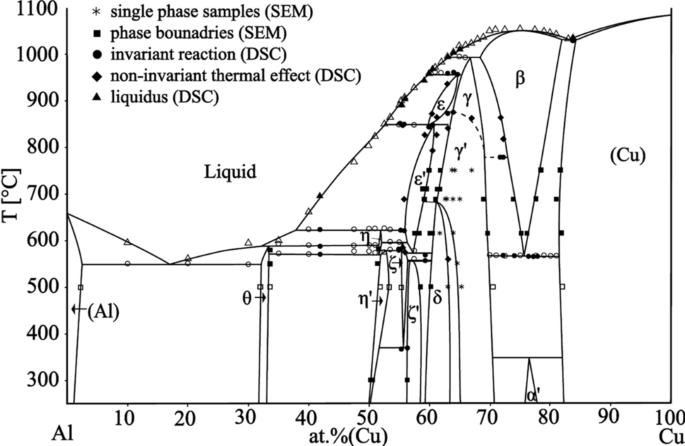
According to them, the Fe 2 W phase is only a metastable transitional phase in the transformation from µ-Fe 7 W 6 to δ-FeW. µ-Fe 7 W 6 is considered a high temperature phase only which occurs in the phase diagram from . The δ-phase was claimed to have the same crystal structure as NiW. ... Full range of the phase diagram. (b) Congruent ...
A congruent phase transformation is "A transformation that the initial phase and the final phase have the same composition", no matter the initial phase is the solid phase or the liquid phase. What is the principal difference between congruent and incongruent phase transformation?
Teach Yourself Phase Diagrams A.6 HRS 03/11/2009 and Phase Transformations DEF.The equilibrium constitution is the state of lowest Gibbs free energy G, for a given composition, temperature and pressure. An alloy in this state shows no tendency to change - it is thermodynamically
For some given binary phase diagram upon heating or cooling, locate temperatures & compositions and write reactions of: Eutectic Eutectoid Peritectic congruent phase transformations 1/2/2015 1:48 PM Dr. Mohammad Abuhaiba, PE 4









![Phase diagrams and phase transformations - [PDF Document]](https://reader021.docslide.net/reader021/html5/20170913/55d30838bb61eb685a8b4857/bg3.png)




0 Response to "40 congruent transformation phase diagram"
Post a Comment