36 exothermic potential energy diagram
Start studying Exothermic Potential Energy Diagram. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
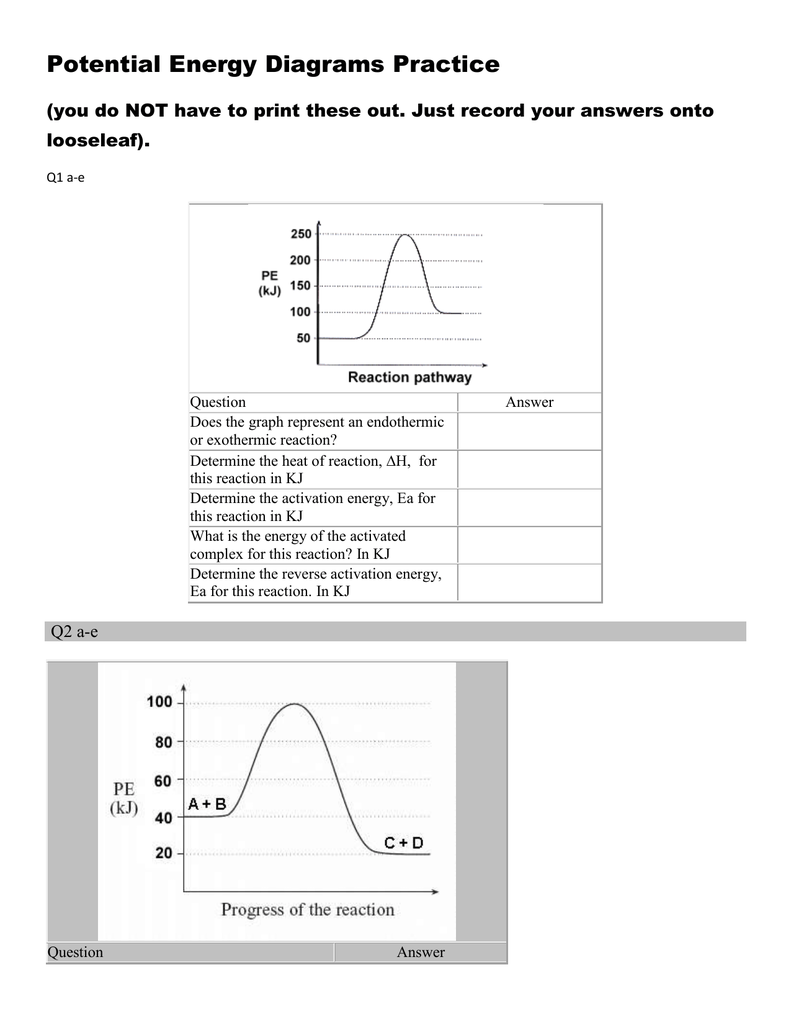
Chemistry*12* Potential*Energy*Diagrams*Worksheet* Name:* Date:* Block:*! USE!THE!POTENTIAL*ENERGY*DIAGRAM!TO!ANSWER!THEQUESTIONS!BELOW:! 1.! Is!the!overall!reaction ...
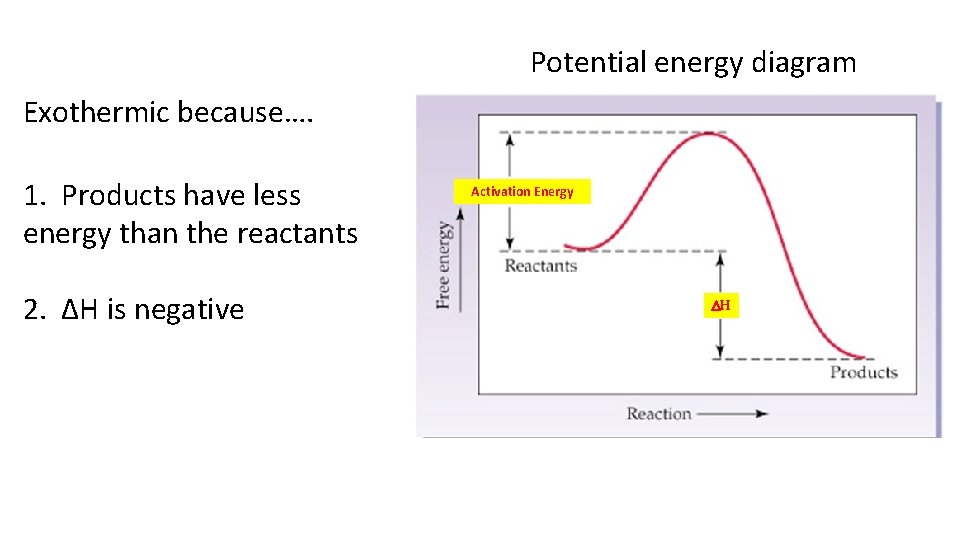
is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the potential energy diagrams. The total potential energy ...

Exothermic potential energy diagram
9 Jul 2019 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
9 Jul 2021 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
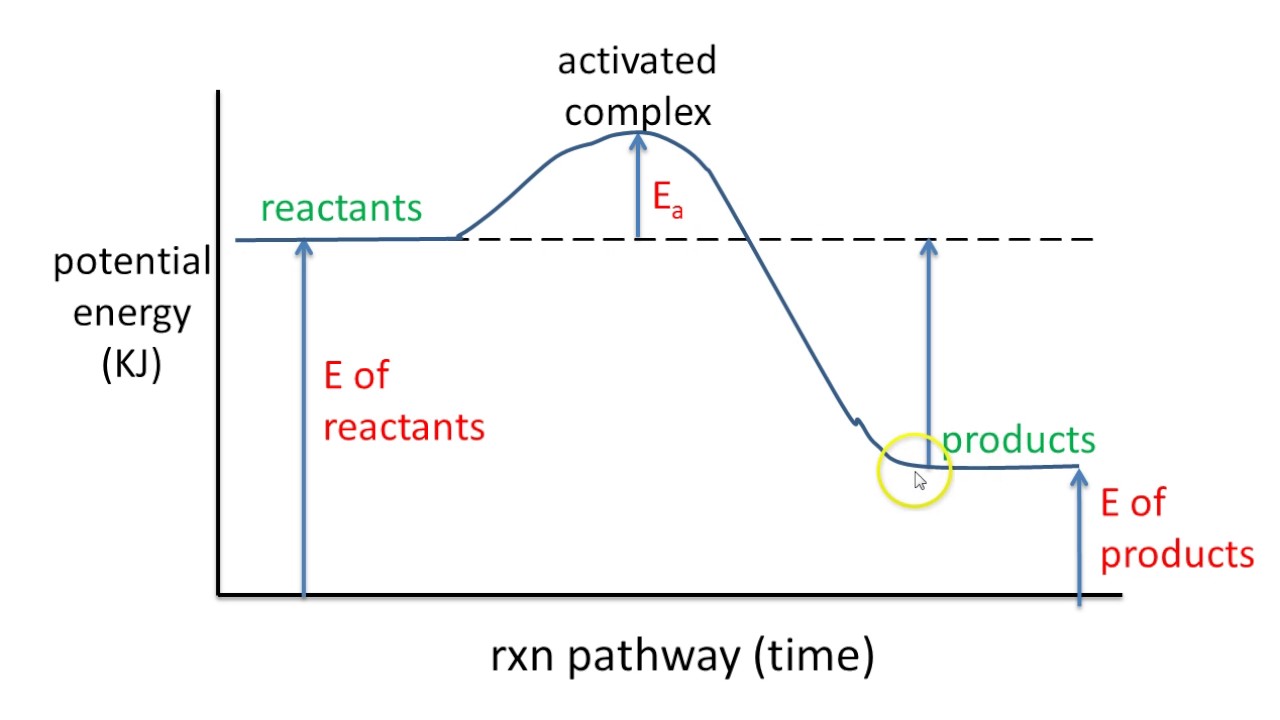
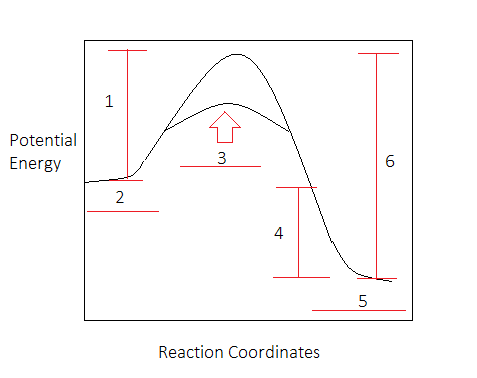
ENERGY DIAGRAM. • A = POTENTIAL ENERGY OF REACTANTS. • B = ACTIVATION ENERGY. • ENERGY REQUIRED TO START THE REACTION. • C = ACTIVATION ENERGY (REVERSE.11 pages
Exothermic potential energy diagram.
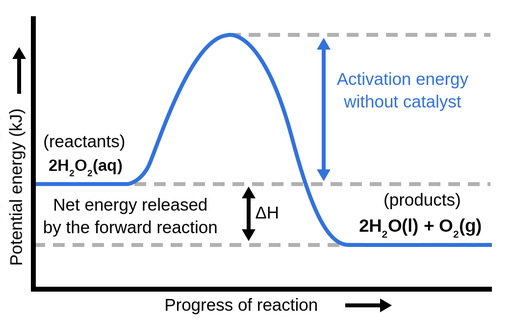
23 Feb 2012 — Potential energy diagrams show how the potential energy changes during reactions from reactants and products. · Exothermic reactions have a ...
The reverse reaction is ____exothermic_____ (endothermic or exothermic). Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what ...
10+ Endothermic Energy Diagram. Endothermic reactionin an endothermic reaction, the products are higher in energy than the an energy diagram can be used to show energy movements in these reactions and temperature can be. An energy level diagram shows whether a reaction is exothermic or endothermic. Energy is absorbed δh = + (net gain).
Potential energy diagrams are used to represent potential energy changes that take place as time progresses during chemical reactions. The potential energy diagrams for the exothermic reaction between substances X and Y is shown below. The reaction progresses from stage 1 (reactants), through stage 2 (activated complex to), to stage 3 (products ...
Both endothermic and exothermic reactions require activation energy. Endothermic Reactions. the reactants have less potential energy than do the products.Question: Answer
the energy released (∆H) Diagram 3 d) a spontaneous exothermic reaction. Diagram 2 depending on the values but looks quite small. To be spontaneous needs to be ≤ 20kJ 2. Draw a potential energy (E p ) diagram for a reaction in which ∆H = 80 kJ/mol and
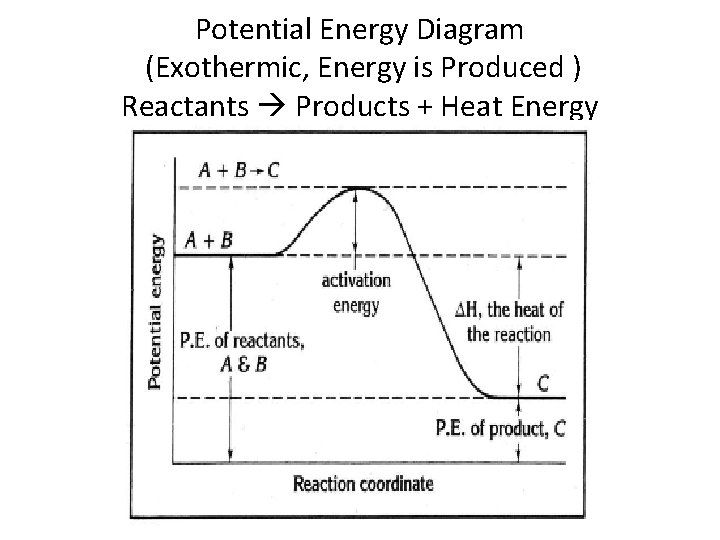
1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
Decrease in kinetic energy - energy released – exothermic Energy level diagrams Chemical Potential Energy The chemical potential energy stored in the bonds gives us a measure of a substances energy level. The higher the energy, the more chemical energy is stored in its bonds. The reactants and products
Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy.
A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change \(\left( \Delta H \right)\) is positive for an endothermic reaction and negative for ...
























0 Response to "36 exothermic potential energy diagram"
Post a Comment