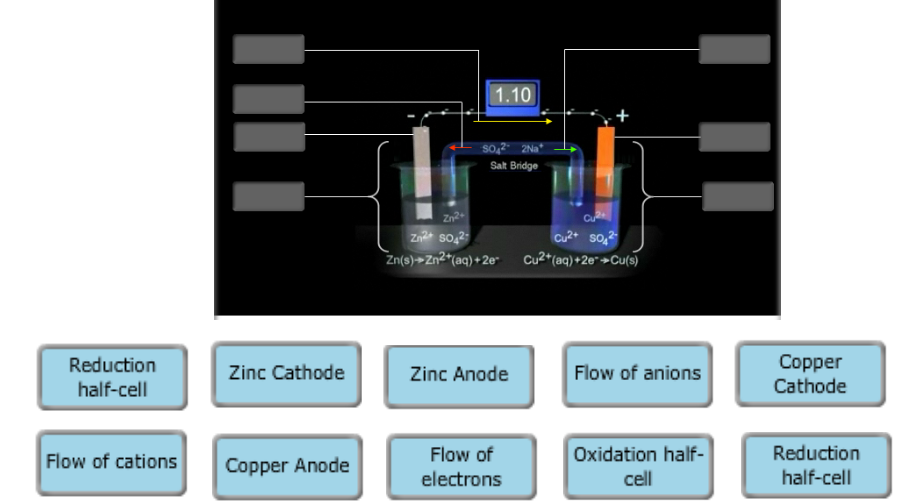
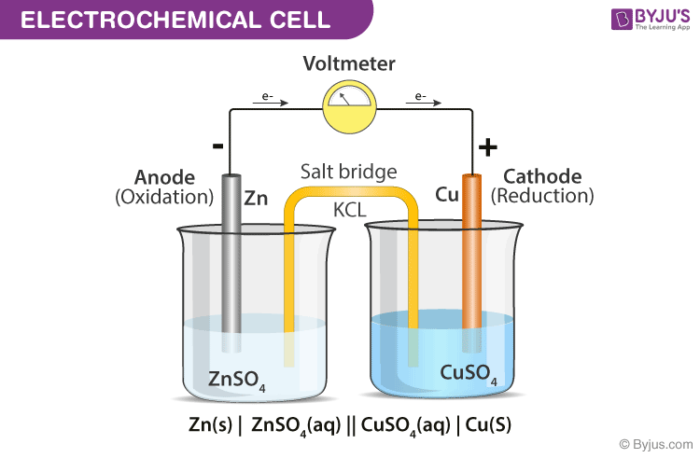
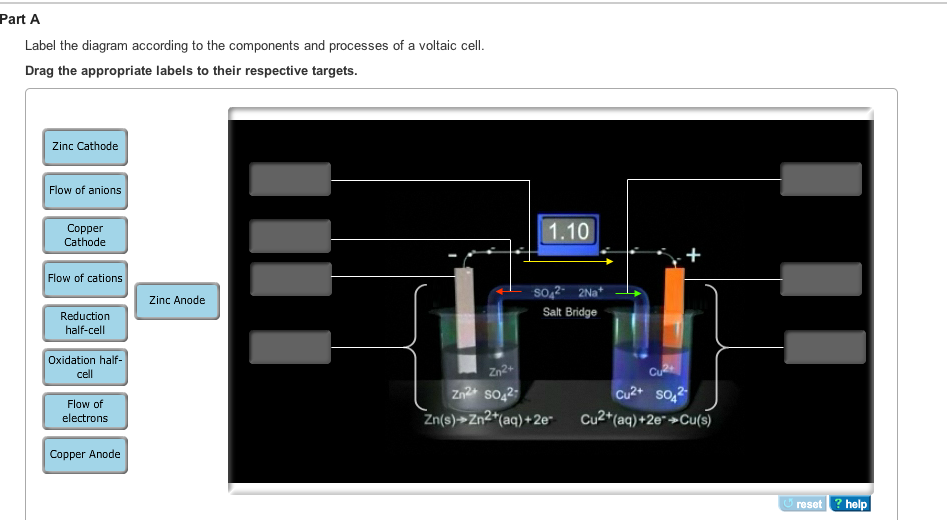
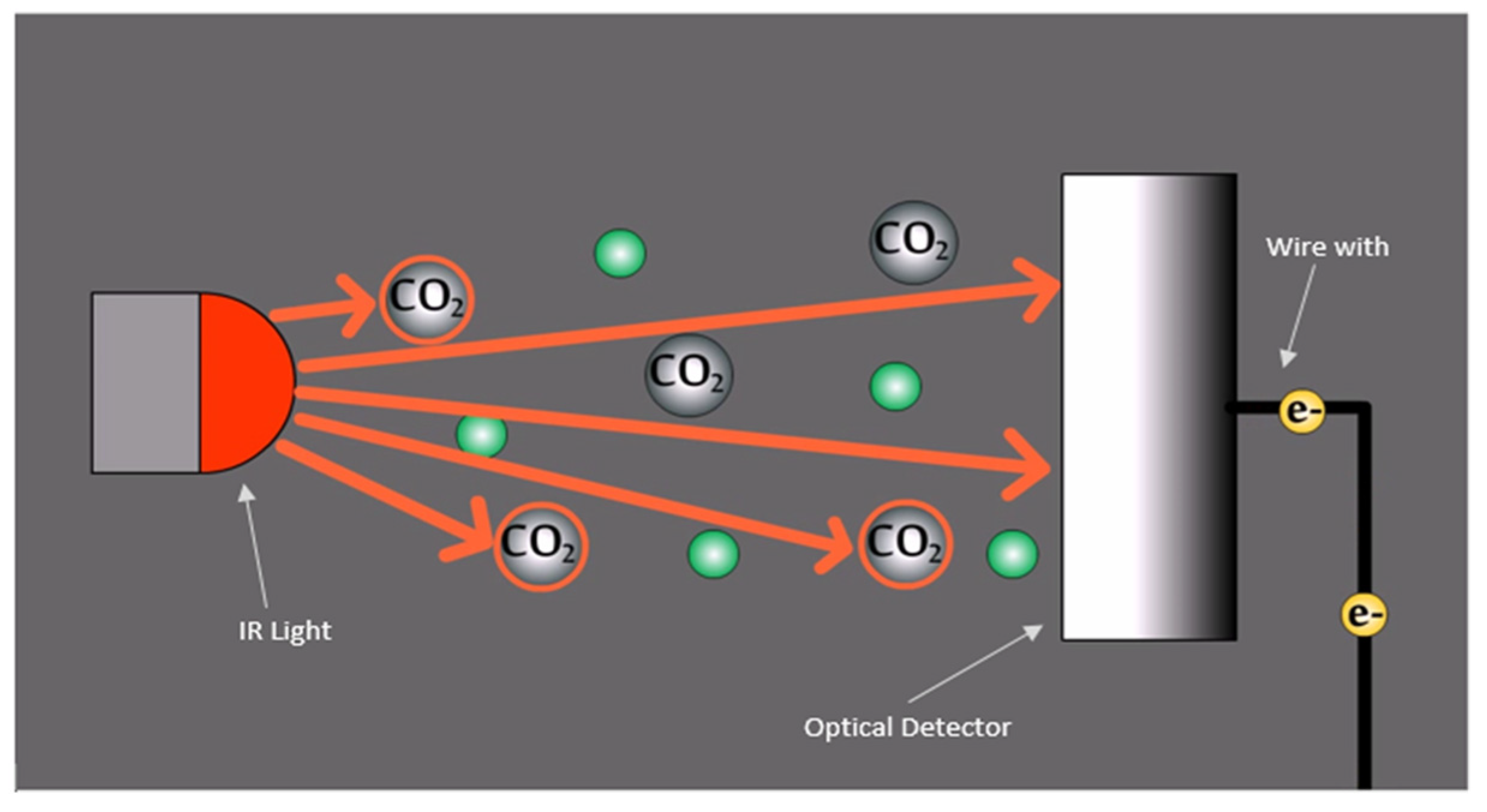
40 label the diagram according to the components and processes of a voltaic cell.
February 4, 2021 - A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. Browse voltaic cell resources on Teachers Pay Teachers, a marketplace trusted by millions of teachers for original educational resources.
Free essays, homework help, flashcards, research papers, book reports, term papers, history, science, politics
Label the diagram according to the components and processes of a voltaic cell.
Donate here: http://www.aklectures.com/donate.phpWebsite video: http://www.aklectures.com/lecture/voltaic-electrochemical-cell-diagramFacebook link: https:// April 10, 2021 - In an electrolytic cell, electrons are forced to flow in the opposite direction. Since the direction is reversed of the voltaic cell, the E0cell for electrolytic cell is negative. Also, in order to force the electrons to flow in the opposite direction, the electromotive force that connects ... August 15, 2020 - Cell notations are a shorthand description of voltaic or galvanic (spontaneous) cells. The reaction conditions (pressure, temperature, concentration, etc.), the anode, the cathode, and the electrode …
Label the diagram according to the components and processes of a voltaic cell.. August 15, 2020 - In redox reactions, electrons are transferred from one species to another. If the reaction is spontaneous, energy is released, which can then be used to do useful work. To harness this energy, the … Achieving Excellence · Register between Oct. 15 and Nov. 7, 2021 When drawing and labeling a diagram of the plasma membrane you should be sure to include: The phospholipid bilayer with hydrophobic 'tails' and hydrophilic '... Cell diagrams for galvanic cells or voltaic cells tutorial with worked example for the Daniell Cell for chemistry students.
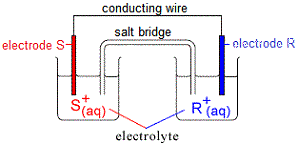
Mr. Abraham's Site Penfield High School 25 High School Drive Penfield, NY 14526 A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous reactions. A common apparatus generally consists of two different metals, each immersed in separate ... In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
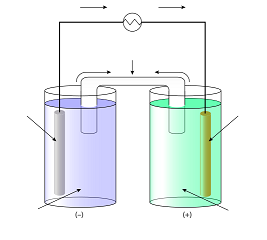
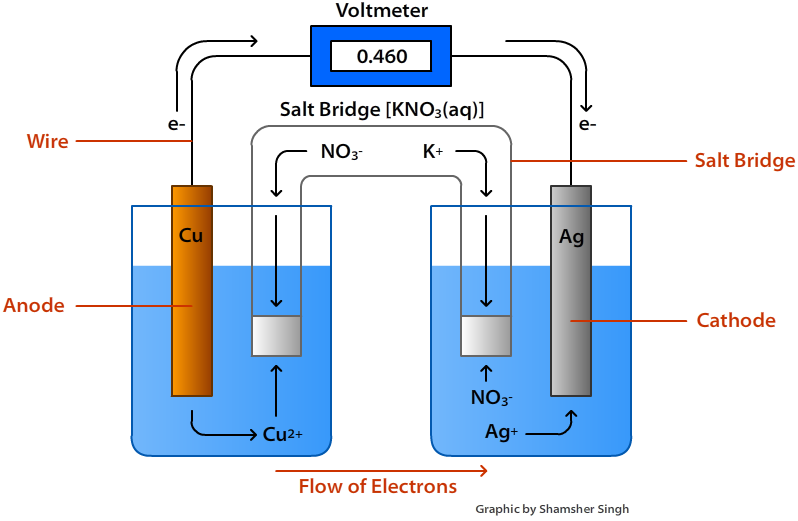
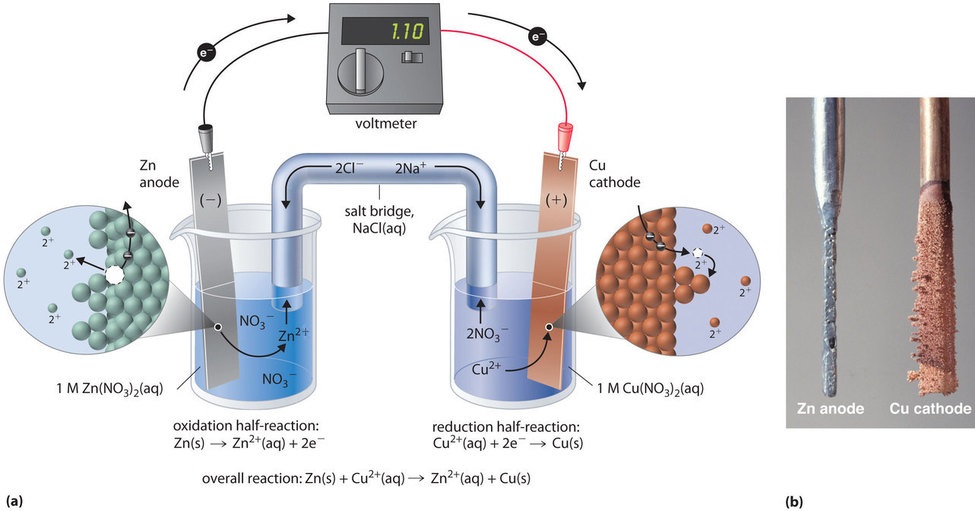
The voltage of the cell can be projected using a voltmeter or a Vernier LabPro interface. A Standard Zn/Cu Voltaic cell is used to show the E°cell generated is the difference in the standard reduction potentials of two half-reactions: Zn2++ 2e- -> Zn and Cu2++ 2e- -> Cu for Zn|Zn2+||Cu2+|Cu ... Galvanic cells, also known as voltaic cells, are electrochemical cells in which spontaneous oxidation-reduction reactions produce electrical energy. In writing the equations, it is often convenient to separate the oxidation-reduction reactions into half-reactions to facilitate balancing the ... August 15, 2020 - Cell notations are a shorthand description of voltaic or galvanic (spontaneous) cells. The reaction conditions (pressure, temperature, concentration, etc.), the anode, the cathode, and the electrode … April 10, 2021 - In an electrolytic cell, electrons are forced to flow in the opposite direction. Since the direction is reversed of the voltaic cell, the E0cell for electrolytic cell is negative. Also, in order to force the electrons to flow in the opposite direction, the electromotive force that connects ...
Donate here: http://www.aklectures.com/donate.phpWebsite video: http://www.aklectures.com/lecture/voltaic-electrochemical-cell-diagramFacebook link: https://

34 Label The Diagram According To The Components And Processes Of A Voltaic Cell Label Design Ideas 2020

35 Label The Diagram According To The Components And Processes Of A Voltaic Cell Labels For Your Ideas

30 Label The Diagram According To The Components And Processes Of A Voltaic Cell Labels Design Ideas 2020

Measurement Techniques To Resolve And Control Population Dynamics Of Mixed Culture Processes Trends In Biotechnology
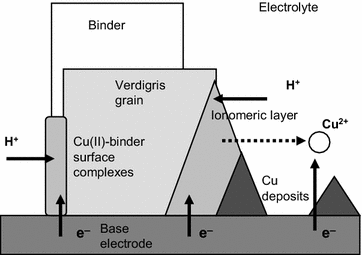
Analyzing Chemical Changes In Verdigris Pictorial Specimens Upon Bacteria And Fungi Biodeterioration Using Voltammetry Of Microparticles Heritage Science Full Text
Draw The Diagram Of Galvanic Cell Which Represents The Following Reaction Sarthaks Econnect Largest Online Education Community

Single Step Label Free Nanowell Immunoassay Accurately Quantifies Serum Stress Hormones Within Minutes Science Advances
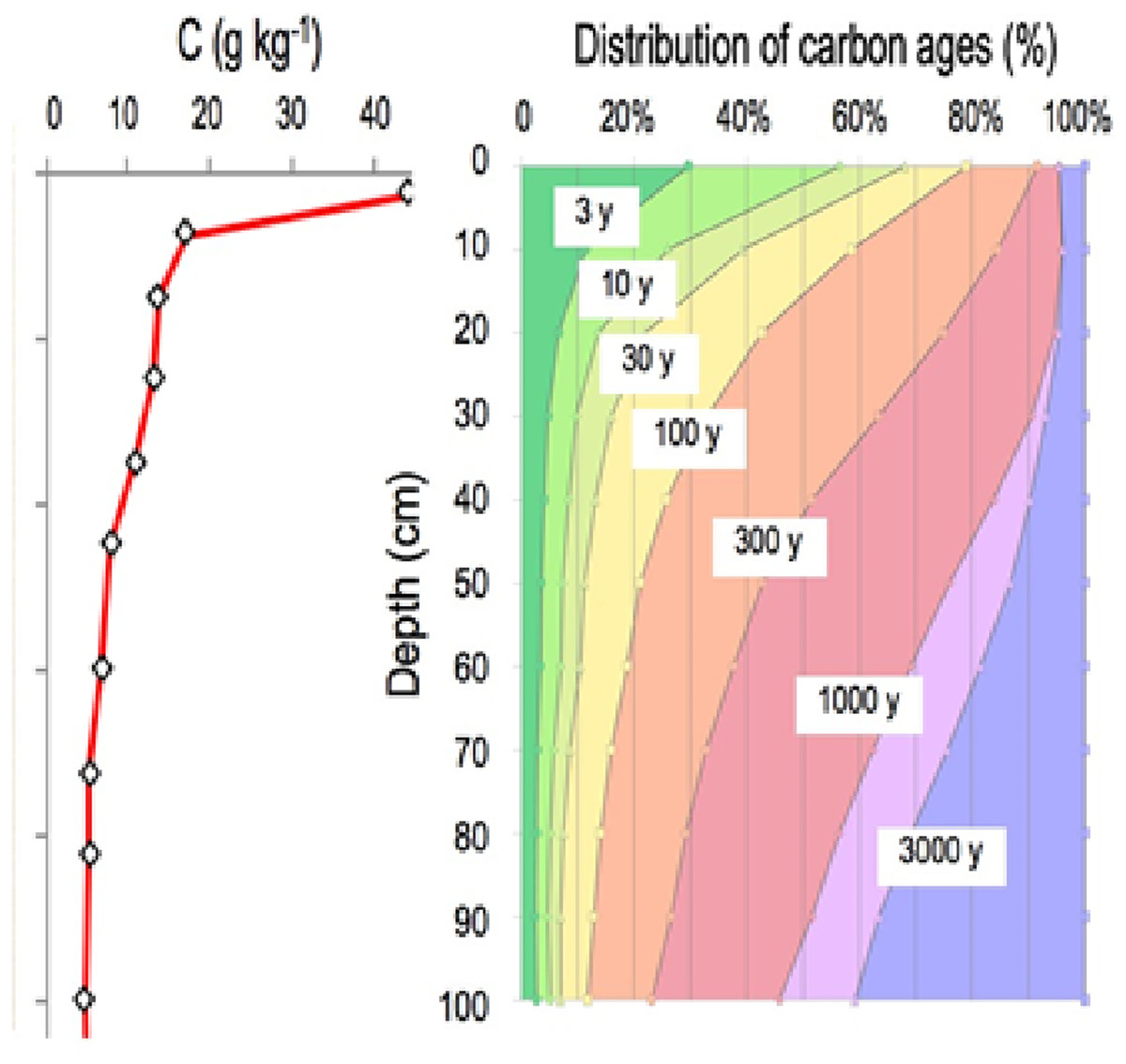
Variation Of Soil Microbial Carbon Use Efficiency Cue And Its Influence Mechanism In The Context Of Global Environmental Change A Review Peerj
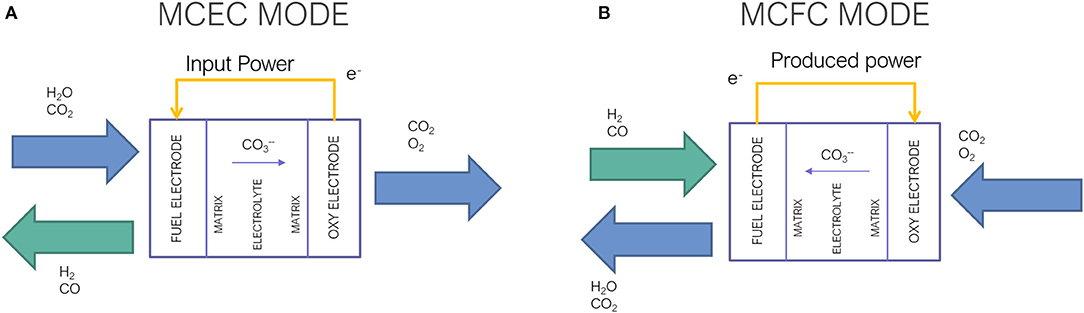
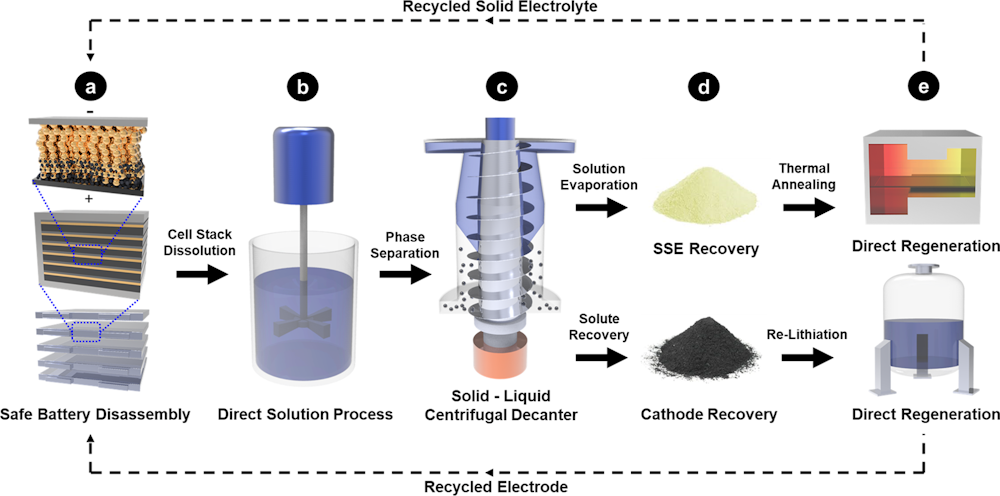
Frontiers A System Integration Analysis Of A Molten Carbonate Electrolysis Cell As An Off Gas Recovery System In A Steam Reforming Process Of An Oil Refinery Energy Research








0 Response to "40 label the diagram according to the components and processes of a voltaic cell."
Post a Comment