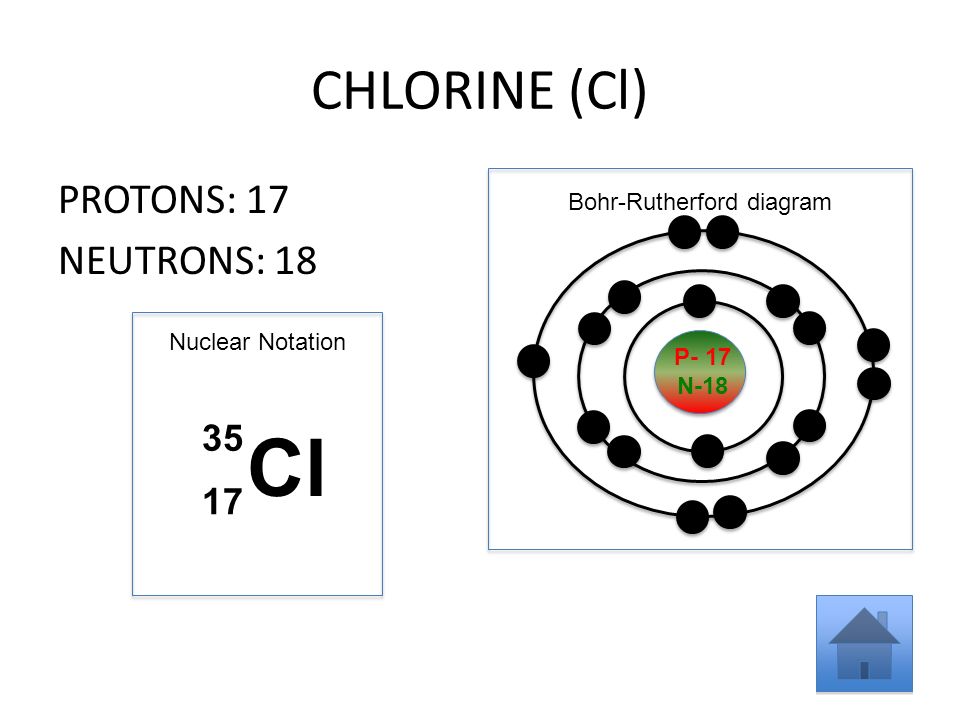
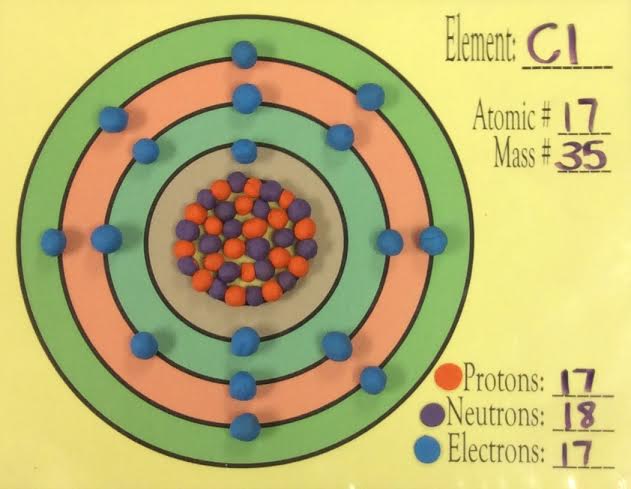
35 bohr diagram for cl
Bohr equation e. Schrödinger equation . c. Pauli exclusion principle ... A student draws the orbital diagram below for the 3d electrons in a V atom. What, if anything, is incorrect about the drawing? {^v}{^ }{ }{ }{ } 3d a. It violates the aufbau principle b. it violates the pauli exclusion principle c. it violates the heisenburg uncertainty principle d. it violates hunds rule e. there is ... Bohr Diagrams So far we have used Bohr diagrams to show how may electrons an element has and how elements bond in ionic compounds. For example here is CaF 2. ... For example, we learned that chlorine has 7 valence electrons. The electron dot symbol for chlorine is shown at the right.
Cl. Bohr Diagram Physical Science Name/Per/Due date_____ Valence Electron Practice. Directions: Give the total number of electrons and the number of valence electrons for each element listed below. Hydrogen 2. Lithium. Beryllium 4. Carbon. Fluorine 6. Neon. Magnesium 8. Chlorine. Arsenic 10. Krypton . Barium 12. Tin. Iodine 14. Aluminum. Directions: Give the element names for the element in ...
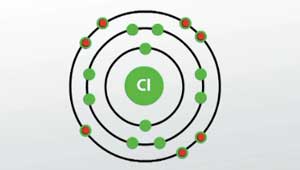
Bohr diagram for cl
Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies.. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. Carbon electron configuration is 1s 2 2s 2 2p 2.Carbon(C) is a p-block element. This article gives an idea about the electron configuration of carbon(C) and the orbital diagram, period and groups, valency and valence electrons of carbon, bond formation, compound formation, application of different principles.. The nucleus is located in the center of the atom. Use the table above to draw the Bohr model diagram for the following atoms and ions. Argon atom 22N Chlorine atom Chlorine ion 19 Potassium atom Potassium ion 3. What do you notice about the arrangement of electrons in the Bohr model of a neon atom, fluorine ion, and a magnesium ion? ALI e ec Levels 4. What would you expect to see with the arrangement of electrons in the Bohr model of an …
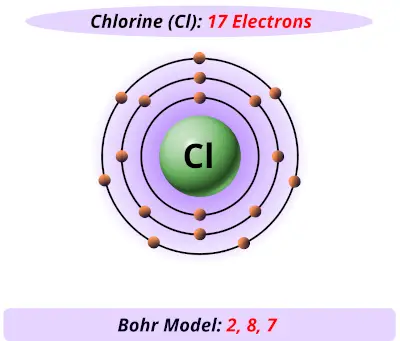
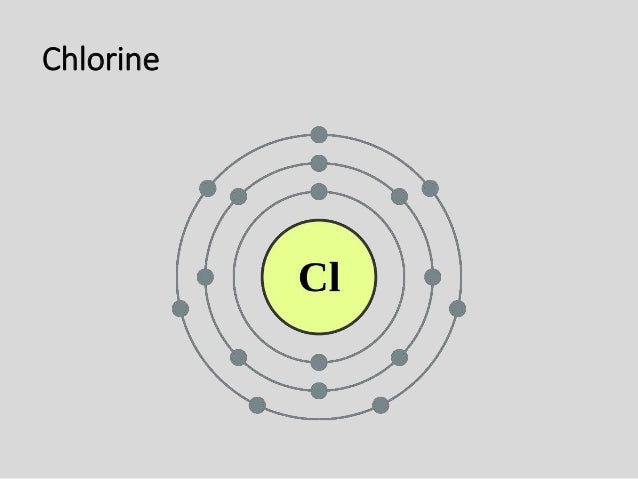
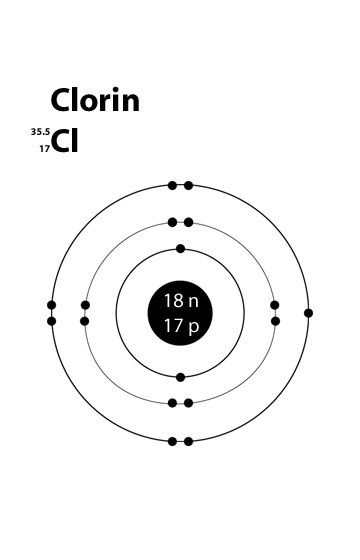
Bohr diagram for cl. Symbol: Cl Atomic Number: 17. Atomic Mass: 35.4527 amu ... Number of Protons/Electrons: 17. Number of Neutrons: 18 ... [Bohr Model of Chlorine] The Bohr Model of Chlorine(Cl) has a nucleus that contains 18 neutrons and 17 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Chlorine contains 7 electrons that also called valence electrons. 02.11.2021 · Draw the following Bohr Model Diagrams (NOTE THEY ARE IONS) Be 2+ Cl-F-N3-Ca2+ K+ Na+ O2-Mg2+ Be2+ S2-Li+ Chem WS 6 Page 1 The Bohr-Sommerfeld model is an extension of the Bohr model. 4) The 2nd energy level can hold up to 8 electrons. Professionally designed block diagram examples and diagramming shortcuts for quick diagramming. Remember that protons and neutrons … 6 Jul 2018 — This is a Bohr model of a chlorine-35 atom. Some Bohr models pair six of the seven electrons in the third (valence) shell.2 answers · No I don't believe so. Explanation: The two electrons in the first shell should be together ...
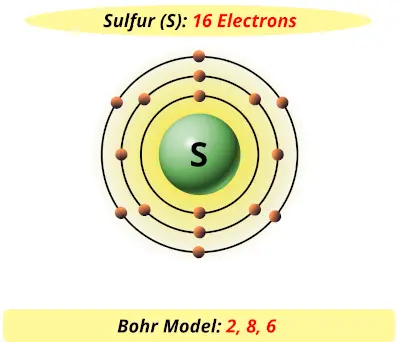
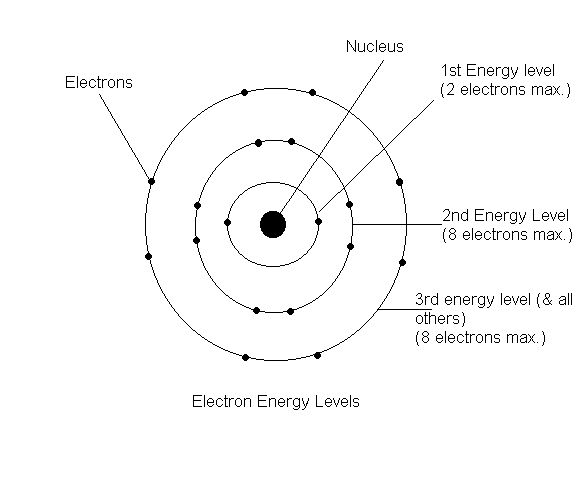
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the The Bohr model was a one-dimensional model that used one quantum number to describe the distribution of electrons in the atom. The only information that was important was the size of the orbit, which was described by the n quantum number. Schrödinger's model allowed the electron to occupy three-dimensional space. It therefore required three coordinates, or three quantum numbers, to … Draw a Bohr Model of Chlorine (Cl) Atomic Number: 17 (# of protons & therefore, Need to know for a Dot Diagram: Element Symbol: Cl Group Number: 17 (# of.Bohr Diagrams • Find out which period (row) your element is in. • Elements in the 1st period have one energy level. • Elements in the 2nd period have two energy levels, and so on. 1:39Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Levels, Lyman & Balmer Series. The ...17 Apr 2014 · Uploaded by Ryan Smith
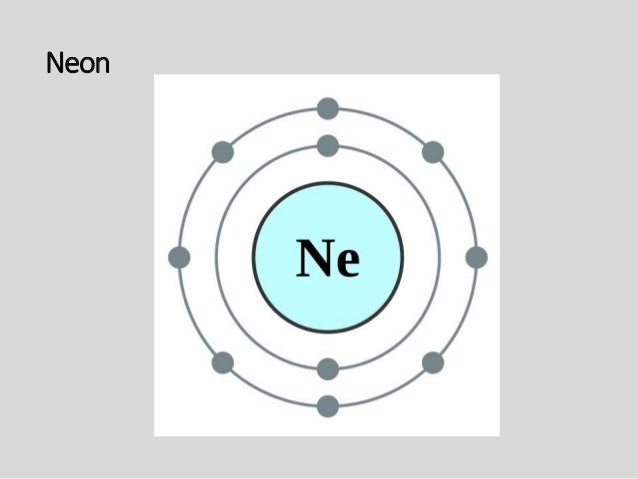
Remember that protons and neutrons are found in the nucleus of the atom and ... Cl. K. Based on what you know about chemical families, which element above ... Chlorine(Cl) Blog; Neon(Ne) Electron Configuration with Full Orbital Diagram. Neon electron configuration is 1s 2 2s 2 2p 6. The symbol for neon is ‘Ne’ and it is an inert element. This article gives an idea about the electron configuration of neon(Ne) and orbital diagram, period and groups, valency and valence electrons of neon, application of different principles. The tenth element in ... 15 Aug 2020 — Bohr diagrams show electrons orbiting the nucleus of an atom somewhat ... In contrast, chlorine and sodium have seven and one electrons in ... Bohr Diagram For Chlorine. A neutral atom has the same number of protons, neutrons, and . This diagram shows the electron shell configuration of a chlorine atom. Draw a Bohr Model of Chlorine (Cl) Atomic Number: 17 (# of protons & therefore, same # of electrons) Atomic Mass: (Atomic mass – Atomic number = # of.
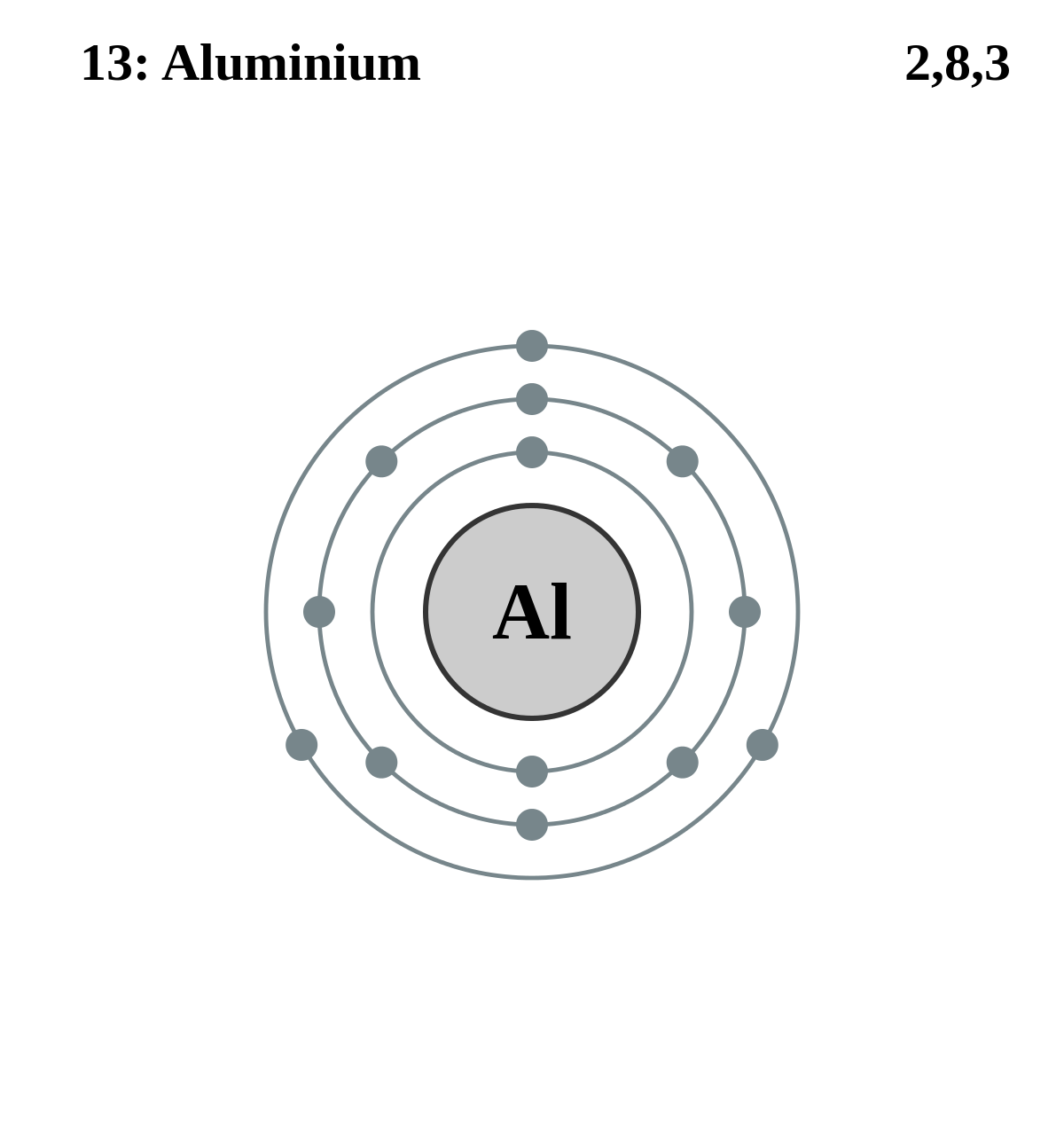
Aluminum has 2 electrons in its first shell, 8 in its second and 3 in its third.Check me out: http://www.chemistnate.com
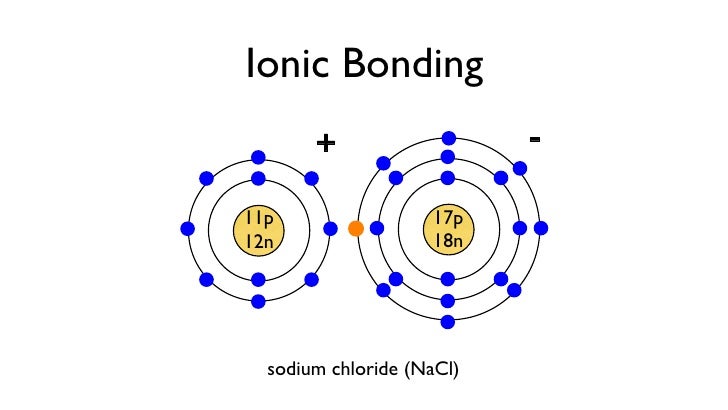
Draw a Dot Diagram. of Chlorine. Cl. Available spot for bonding!! Cl ¯ Draw a Bohr Model of Sodium (Na) Atomic Number: 11 (# of protons & therefore, same # of electrons) Atomic Mass: 22.99 (Atomic mass – Atomic number = # of neutrons) Alkali: Group 1 (# of valence electrons) Period 3
The halogens (F, Cl, Br etc.) are one electron short of a valence shell octet, and are among the most reactive of the elements (they are colored red in this periodic table). In their chemical reactions halogen atoms achieve a valence shell octet by capturing or borrowing the eighth electron from another atom or molecule. The alkali metals Li, Na, K etc. (colored violet above) are also ...
Draw a Bohr Model of Chlorine (Cl). Atomic Number: 17. (# of protons & therefore, same # of electrons). Atomic Mass: 35.453.
Using The Main Group Elements Of The Periodic Table To Draw Bohr Rutherford Diagrams He Ppt Download
Chlorine has 2 electrons in its first shell, 8 in its second and 7 in its third.Check me out: http://www.chemistnate.com
Use the table above to draw the Bohr model diagram for the following atoms and ions. Argon atom 22N Chlorine atom Chlorine ion 19 Potassium atom Potassium ion 3. What do you notice about the arrangement of electrons in the Bohr model of a neon atom, fluorine ion, and a magnesium ion? ALI e ec Levels 4. What would you expect to see with the arrangement of electrons in the Bohr model of an …
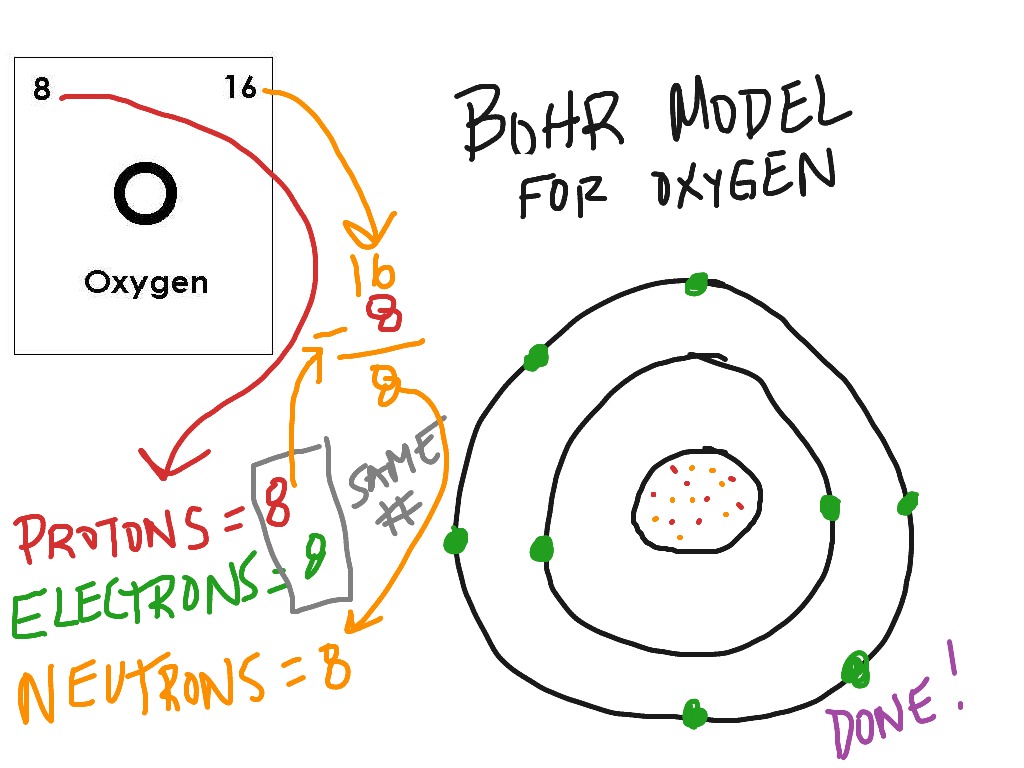
Carbon electron configuration is 1s 2 2s 2 2p 2.Carbon(C) is a p-block element. This article gives an idea about the electron configuration of carbon(C) and the orbital diagram, period and groups, valency and valence electrons of carbon, bond formation, compound formation, application of different principles.. The nucleus is located in the center of the atom.
Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies.. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table.

Draw A Bohr Model For Carbon Dioxide And Sodium Chloride Include Chemical Symbol Snd Either Label Homeworklib

Bohr Model Atom Zirconium Bohr Radius Chemical Element Structural Drawing Chemical Element Text Logo Png Pngwing





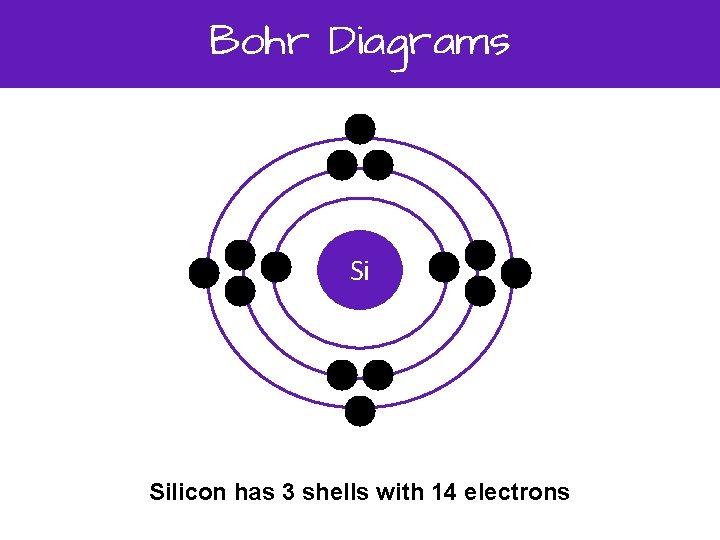







0 Response to "35 bohr diagram for cl"
Post a Comment