39 molecular orbital diagram practice worksheet
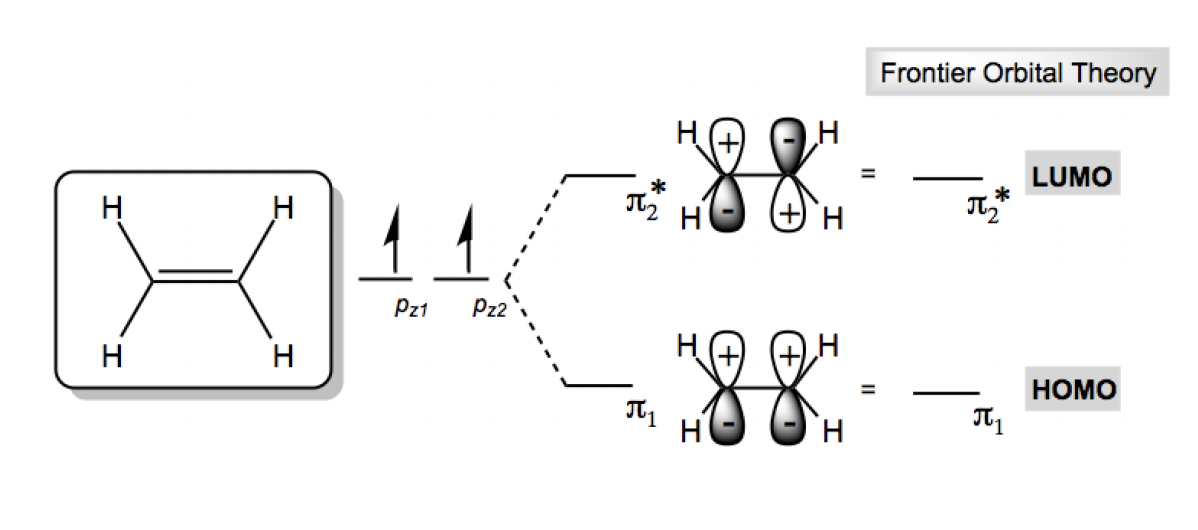
Displaying top 8 worksheets found for - Orbital. The number of molecular orbitals created by. Using arrows show how the following orbitals will fill with electrons. What is the ml values for the following types of orbitals. Worksheet 14 - Hybridization When atoms bond to form molecules they use molecular orbitals. 2. Draw the four π-molecular orbitals for the molecule butadiene, drawn below. Label . the four orbitals as bonding, non-bonding or antibonding. Which of the four orbitals . are occupied? Which is the LUMO and which is the HOMO? 3. Draw the six π-molecular orbitals for benzene, label them as bonding non-bonding or . antibonding. Which are ...
Lesson Worksheet: Molecular Orbital Theory. In this worksheet, we will practice describing the shapes, energies and electron occupancies of bonding, nonbonding, and antibonding molecular orbitals. An sp 2 hybridized atom in a molecule forms 𝜎 bonds to three other atoms of the same element. There are no other bonds in the molecule.
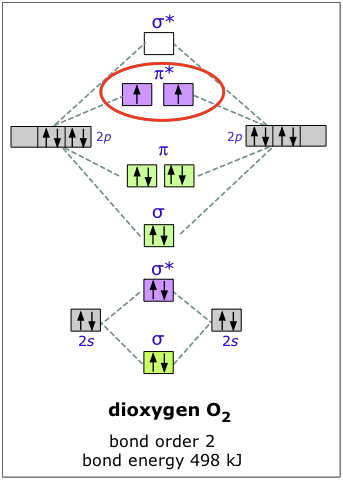
Molecular orbital diagram practice worksheet
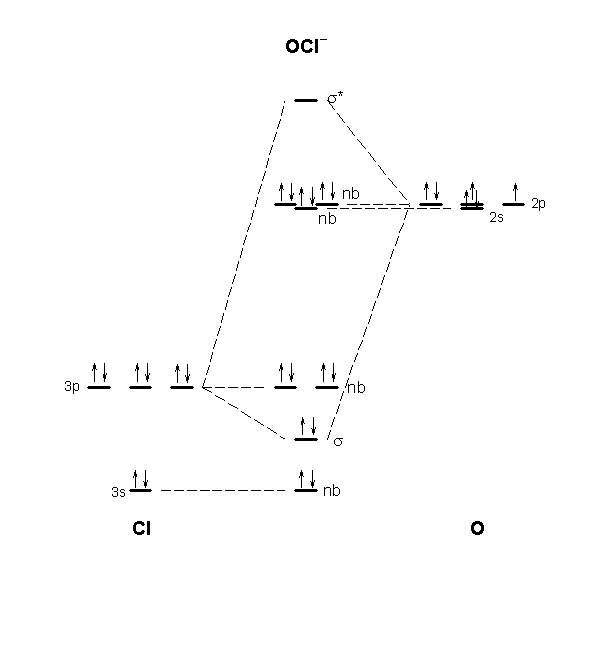
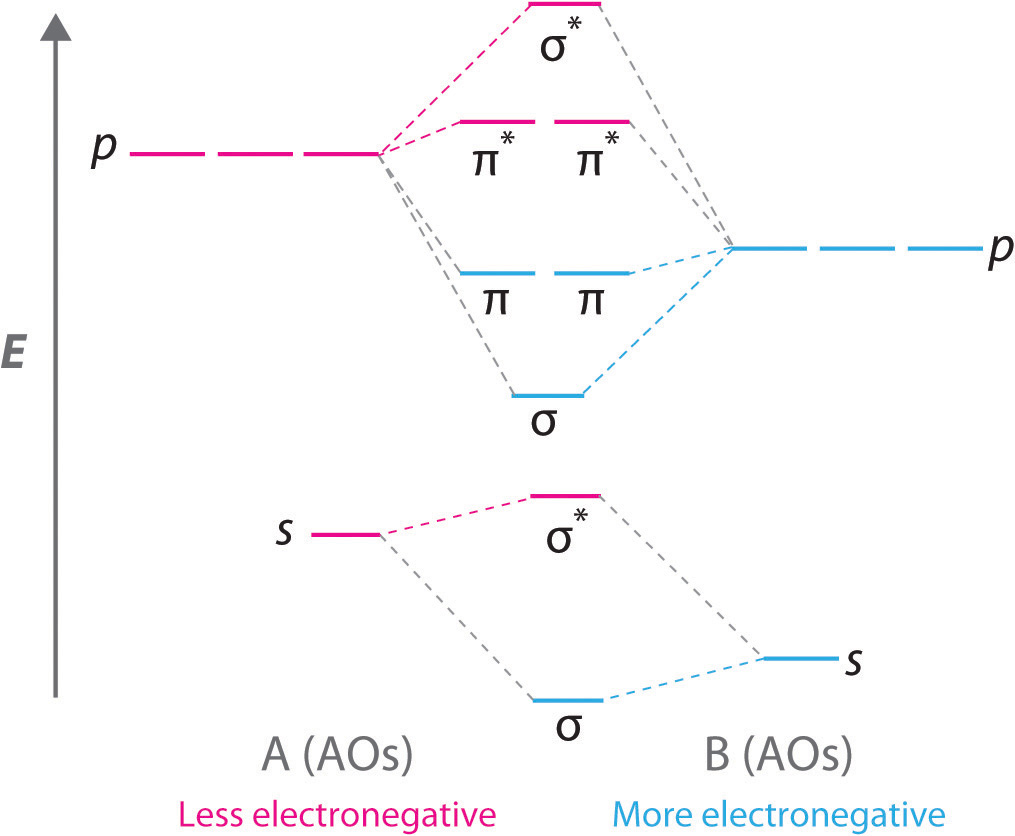
The molecular orbital diagram for ClO - is given below: The basis orbitals for Cl are 3s and 3p and for O are 2s and 2p. Z* for O 2s and 2p orbitals are similar so the AOs start at nearly the same energy. For the Cl 3s and 3p orbitals the two Z* values are quite different so the initial energies are more separated. MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine MO Diagram: 2s. 2pz b. The energy level diagrams for CH2 and BeH2 feature the same orbital interactions. One difference is that the different number of ...29 pages
Molecular orbital diagram practice worksheet. Print Molecular Orbital Theory: Tutorial and Diagrams Worksheet 1. What happens when both of the orbitals in a molecule are in phase, either both positive or both negative, and the electrons in ... Showing top 8 worksheets in the category orbital diagrams. These problems are for practice in drawing your molecular orbital diagrams molecular electron configurations and determining bond order. Draw the molecular orbital diagram for n2 ion and calculate the bond order. Western connecticut state university molecular orbital theory. Rationalize why it is trigonal pyramidal by comparing the appropriate molecular orbital diagrams. 26 See the Walsh Diagram in ab for both confirmations,. -LUMO ...8 pages Chapter 11 Answers Practice Examples 1a. There are three half-filled 2p orbitals on N, and one half-filled 5p orbital on I. Each half-filled 2p orbital from N will overlap with one half-filled 5p orbital of an I. Thus, there will be three N—I bonds. The I atoms will be oriented in the same direction as the three 2p orbitals of N: toward the x ,y, and z-directions of a Cartesian coordinate ...
In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ... However, the diagram will still yield the correct bond order and magnetic behavior for these molecules. 1. Refer to the Molecular Orbital diagram above.2 pages Write a complete electronic configuration and orbital diagram for zirconium, Zr. Molecular Orbital Theory Worksheet 1. Sketch the molecular orbital diagram of Hz-. Write the electron configuration of the ions in terms of its MOu0026#39;s. Worksheet 2 Notes Dr. Richard Nafshun - Welcome to the OSU ... Day 8 Molecular Orbital Theory Part 3 1 Inorganic Chemistry with Doc M. Day 8. Molecular Orbitals: Symmetry adapted linear combinations, SALCs Topics: 1. Symmetry adapted linear combinations (SALC) of bonding group atomic orbitals 2. The 10-Step approach to making MO diagrams via symmetry considerations 3. Molecular orbital diagram for bent H2O 4.
About this quiz & worksheet. Answer the following questions using your unit 3 notes. The following is an orbital diagram for selenium. Practice energy diagrams for molecular orbital theory. An orbital diagram uses boxes with arrows to represent the electrons in an atom. Each box in an orbital diagram represents an . In chemistry molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule. ... Practice energy diagrams for molecular orbital theory. Calculate the number of bonding and ... Molecular orbital Diagram Practice. molecular orbital diagrams of diatomics worksheet in chemistry molecular orbital mo theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms but are treated as moving under the influence of the nuclei in the whole molecule in this theory each ... A Brief Introduction To Molecular Orbital Theory Of Simple. Drawing 3d Molecules Schematic Examples Of 0d 1d And 3d. What Is The Molecular Orbital Diagram Of O2 And F2 Quora. Molecular Orbital Theory Boundless Chemistry. Che 110 Molecular Orbital Practice Problems Answers Pdf Molecular. Chapter 6 5 Delocalized Bonding And Molecular Orbitals ...
A molecular orbital diagram explains chemical bonds within molecules based on the union of atomic orbials. Concept #1: A heteronuclear diatomic molecule is composed of two different elements covalently bonded together. Concept #1: A heteronuclear diatomic molecule is composed of two different elements covalently bonded together. play-rounded-fill.
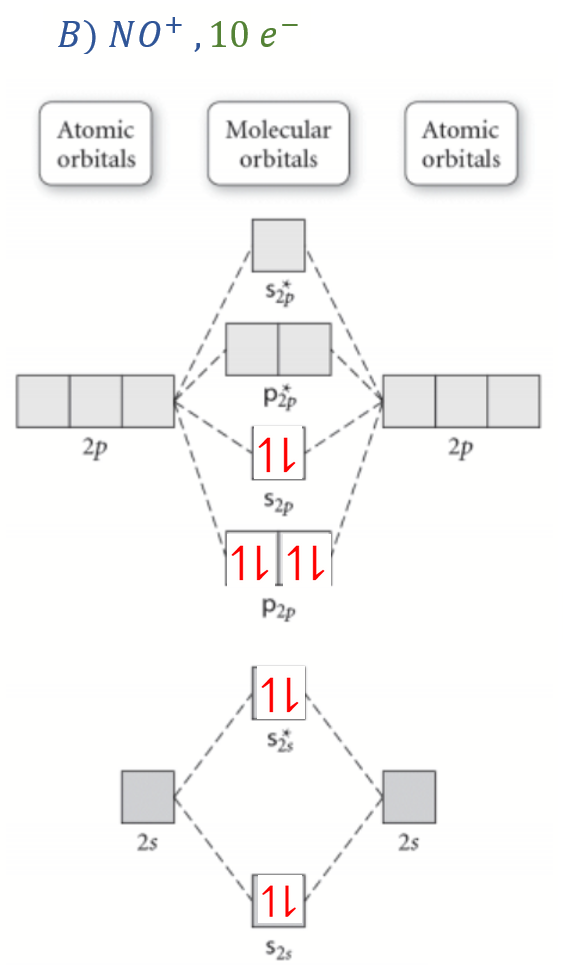
Answers to Practice Test Questions 3 . Molecular Orbital Theory: Heteronuclear Diatomic Molecules . 1. (a) 1The electron configuration for 𝐻𝐻 is 1𝑠𝑠, so 𝐻𝐻 has 1 valence electron. ... The MO diagram shows three pairs of nonbonding electrons in MOs localized on Cl. The two bonding theories therefore give the same picture of ...
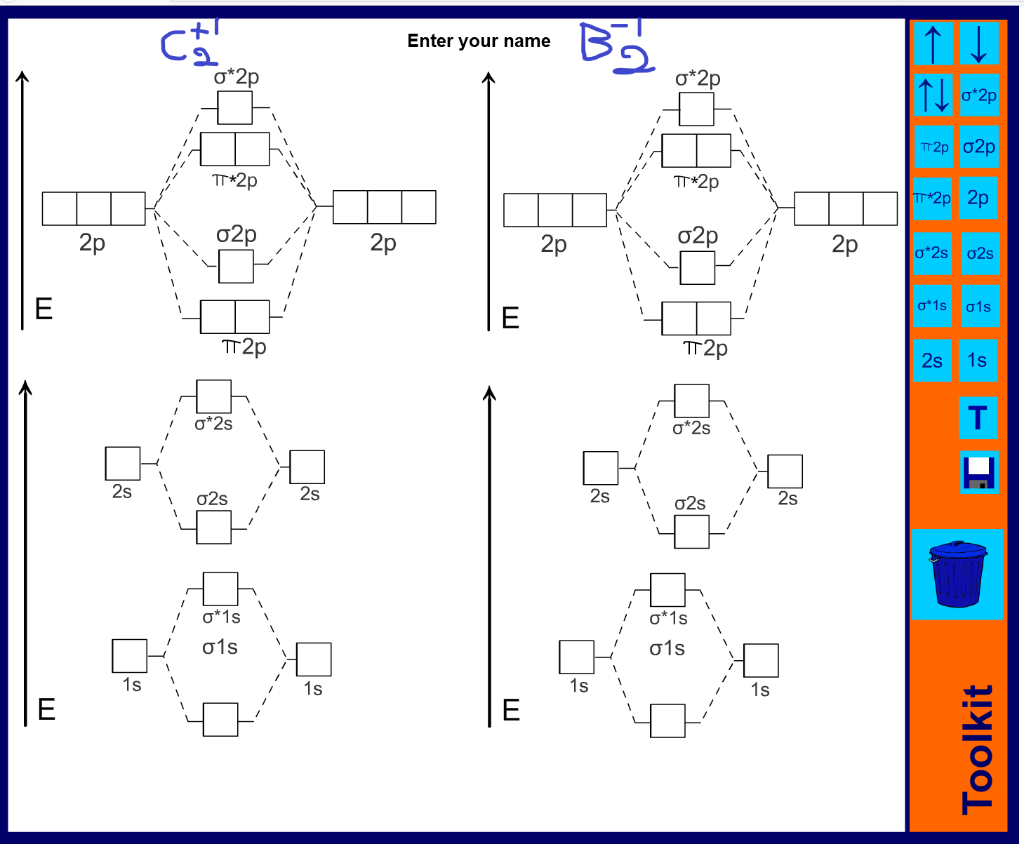
Draw the molecular orbital diagram for B 2. The number of unpaired electrons in the B 2 molecule is _____. (a) zero (b) 1 (c) 2 (d) 3 (e) 4 8. Which one of the following statements is false? (a) Valence bond theory and molecular orbital theory can be described as two different views of the same thing.
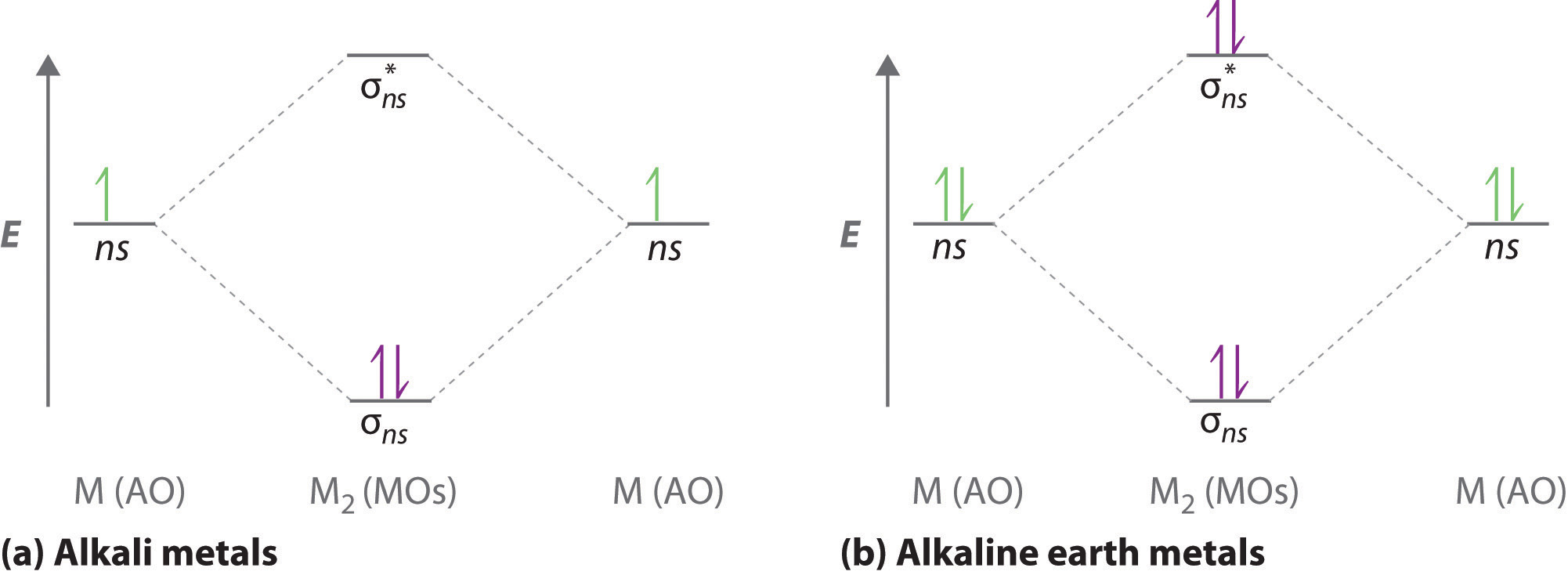
LCAO MO Energy Diagram for H2 Energy H-H ∆E1 ∆E2 • ∆E2> ∆E1, so the antibonding orbital is always more anti-bonding than the bonding orbital is bonding H2molecule: two 1s atomic orbitals combine to make one bonding and one antibonding molecular orbital. Ha Hb
together to produce a sigma molecular orbital [σ = (1sa + 1sb)]. Since the electrons in this orbital are more stable than on the individual atoms, this is referred to as a bonding molecular orbital. A second molecular orbital is also created, which we simplistically show as a subtraction of the two atomic 1s orbitals [σ* = (1sa - 1sb)]. This ...
All about nitrogen! a. Fill in the molecular orbital diagram below for the N2 molecule. b. Draw “cartoon” representations for each of the occupied orbitals.2 pages
Show the molecular orbital diagram for the He2 molecule. What is the helium-helium bond order? How many unpaired electrons are present? Are your results ...10 pages
Chemistry orbital diagram worksheet molecular orbital diagram worksheet orbital diagram practice worksheet orbital diagram worksheet orbital diagram worksheet 1 orbital diagram worksheet 5 5 orbital diagram worksheet 5 5 answers orbital. And that actinium ac is the first element in the 5f block. Is 2s lectron is 4s on 2s a o o gurations or ome ...
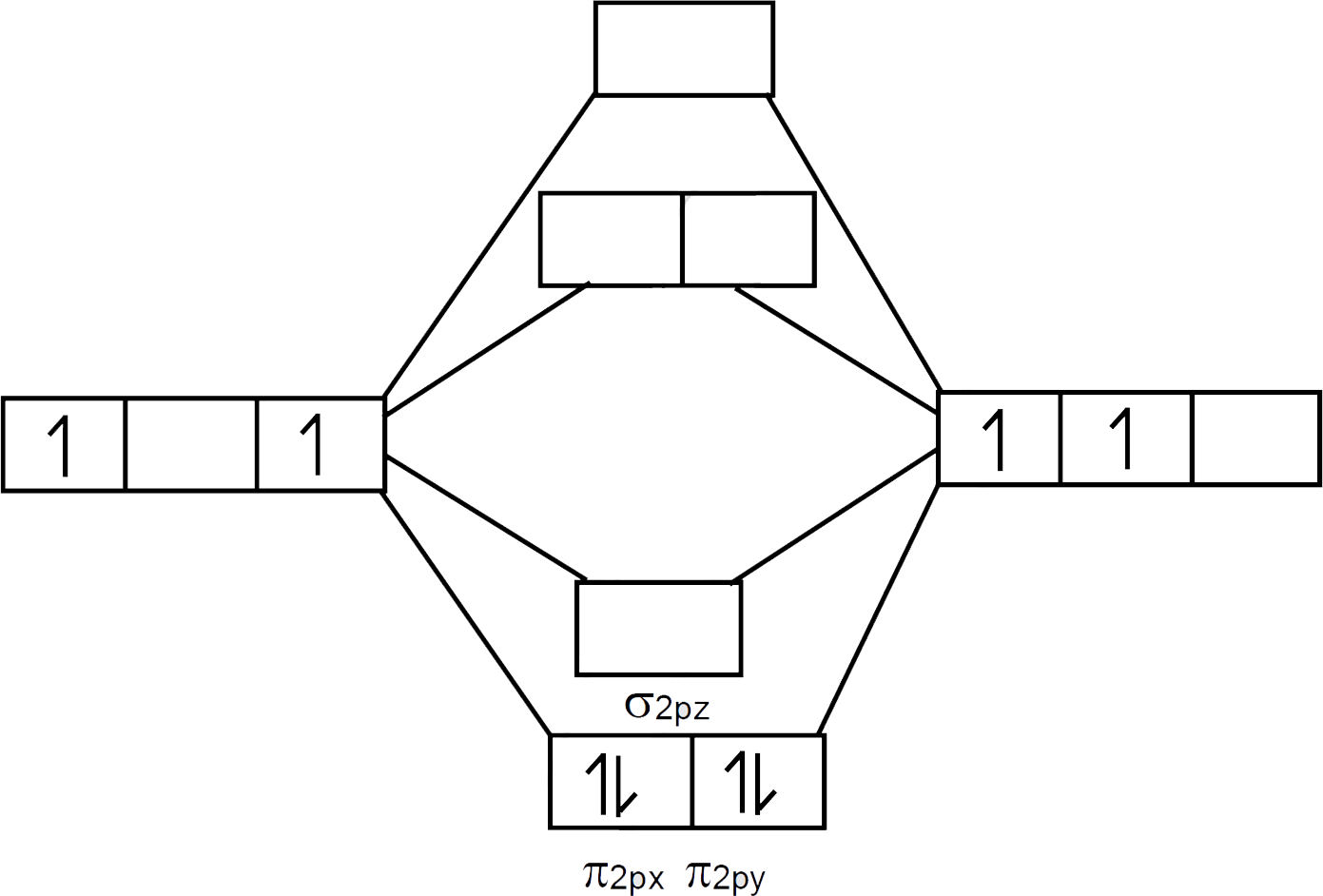
molecular orbital energy-level diagram for the NO molecule. We assume that orbital order is the same as that for N2. The bond order is 2.5. Figure 9.42: The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure 9.43: A partial molecular orbital energy-level diagram for the HF molecule.
Molecular orbital diagram practice worksheet •Molecular orbital theory (MO) - a molecule is formed by the overlap of atomic orbitals to form molecular orbitals, electrons are then distributed into MOs. Write orbital filling diagrams electron configurations and electron dot diagrams. 30 m B) 1.
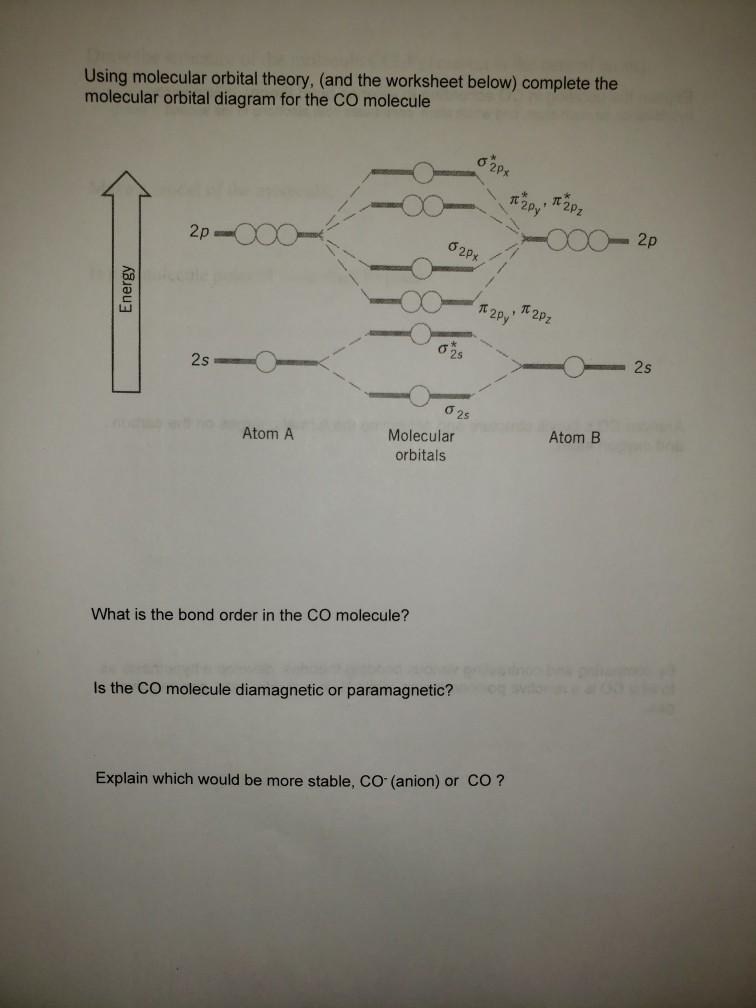
The following molecular orbital diagram may be used for the following problems. For oxygen and fluorine, the σ 2p orbital should be lower in energy than the π 2p. However, the diagram will still yield correct bond order and magnetic behavior for these molecules.
CHEM 2000 Exercises and Practice Test Questions. Exercises are short focused sets of practice questions that can be printed and used as worksheets. Each Exercise focuses on a single concept or skill. You should complete Exercises immediately after the concept or skill is discussed in class to ensure that you fully understand it so that you do ...
6. There is one p orbital on boron but there is no adjacent atom with another p orbital. Add it to the molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1.
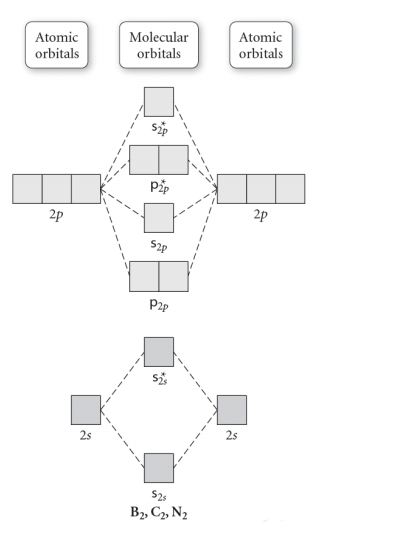
A molecular orbital energy level diagram just shows the energy levels in the molecule. Frequently, but not always, energy level diagrams are shown without any pictures of the orbitals, in order to focus attention on the energy levels, which in a fundamental way are the most important part of the picture.
Molecular Orbital Diagram Practice. February 15, 2015 . By Marsha Massey. University of Sydney has created a practice website for reviewing different parts of molecular orbital diagrams. Using this resource you can add pieces to pre-drawn MO diagrams for over 20 different molecules.
Molecular orbital Diagram Practice. molecular orbital diagrams of diatomics worksheet in chemistry molecular orbital mo theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms but are treated as moving under the influence of the nuclei in the whole molecule in this theory each molecule has a set of molecular orbitals ...
Refer to the MO Diagrams. Assuming that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a hero nuclear diatomic molecule, ...9 pages
MO Diagram: 2s. 2pz b. The energy level diagrams for CH2 and BeH2 feature the same orbital interactions. One difference is that the different number of ...29 pages
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
The molecular orbital diagram for ClO - is given below: The basis orbitals for Cl are 3s and 3p and for O are 2s and 2p. Z* for O 2s and 2p orbitals are similar so the AOs start at nearly the same energy. For the Cl 3s and 3p orbitals the two Z* values are quite different so the initial energies are more separated.


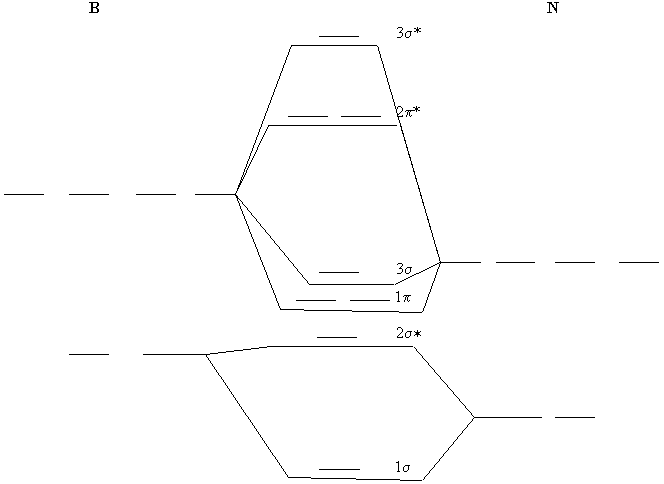













0 Response to "39 molecular orbital diagram practice worksheet"
Post a Comment